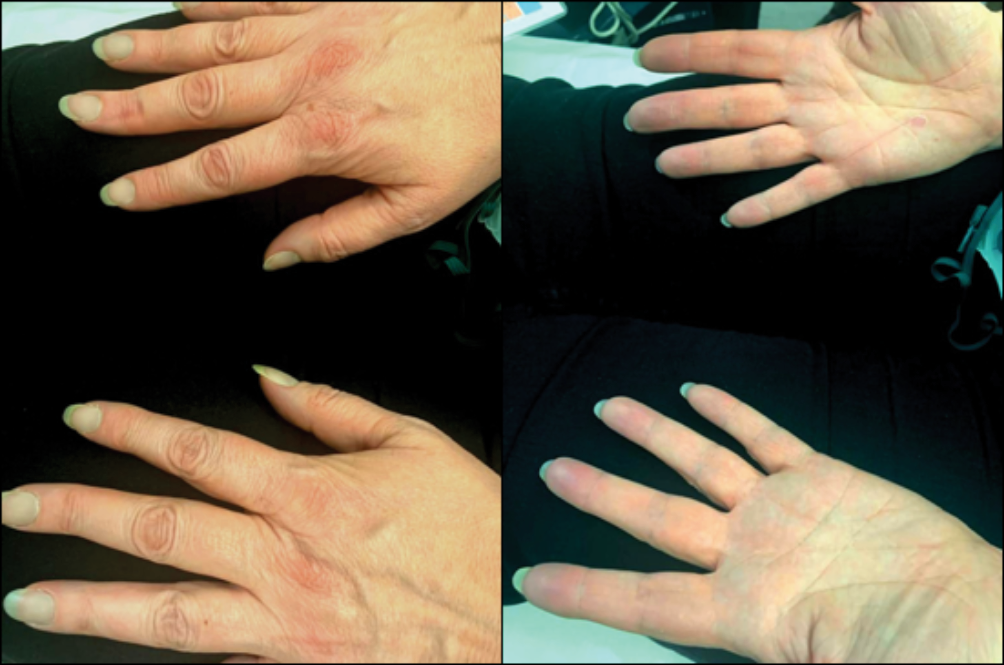
A 48-year-old female presented to the Emergency Department with a two-week history of increasing fatigue. On examination, she had blue-grey central and peripheral cyanosis with tachypnoea and an oxygen saturation of 85% on room air (Figure 1). She was placed on high-flow oxygen, but her oxygen saturation on the pulse oximeter only marginally improved to 90%. Arterial blood gas (ABG) on room air showed pH 7.66 (7.36–7.47), PaCO2 2.89 kPa (4.60–6.40 kPa), PaO2 13.7 kPa (10.6–14.6 kPa), HCO3– 26.6 mmol/L (22–28 mmol/L), base excess 1.2 mmol/L (–2 to +1 mmol/L), SaO2 96.4% (>94%), FO2Hb 81.3% (>94%) and methaemoglobin 15.9% (<1%). She had a background history of hidradenitis suppurativa (HS) and asthma. She had been on dapsone for the last four years for HS and the dosage had been recently increased by her dermatologist from 100 mg to 200 mg once daily. Additionally, her records revealed a persistently elevated reticulocyte count for the past four years and unexpectedly high oxygen demands during a recent surgical procedure. These findings led to the diagnosis of dapsone-induced methaemoglobinaemia.
Figure 1 Picture of patient with peripheral cyanosis at presentation to hospital
The patient was asked to stop dapsone and discharged the same day. She returned to the outpatient clinic one week later with resolution of both her symptoms and clinical signs of cyanosis. Her oxygen saturation was 98% on air and an ABG revealed normal arterial oxygen saturation (SaO2), fractional oxyhaemoglobin (FO2Hb) and methaemoglobin levels.
Methaemoglobinaemia is defined as an abnormal increase in methaemoglobin levels, i.e. >1–2% of total haemoglobin.1 It arises due to oxidation of one or more haem molecules within haemoglobin from the reduced ferrous state to the ferric state. High methaemoglobin levels cause left-shift of the oxygen–haemoglobin dissociation curve and impaired oxygen delivery to tissues. Whilst small amounts of methaemoglobin are produced constantly, the enzymes cytochrome-b5 reductase and NADPH methaemoglobin reductase maintain methaemoglobin levels below 1%.2 When the rate of methaemoglobin formation exceeds that of reduction, tissue hypoxia occurs. This can arise due to congenital disorders, e.g. genetic defects in haemoglobin structure/metabolism, or acquired causes from exposure to external oxidising agents, e.g. food additives, workplace chemicals (e.g. aniline dyes) or drugs such as dapsone (Table 1).2 Haemolysis is also well-documented with dapsone therapy and is associated with the presence of methaemoglobinaemia.3
Table 1 Drugs causing methaemoglobinaemia2
|
|
|
Benzocaine
Prilocaine
Sulphonamides
Dapsone
Ciprofloxacin
Trimethoprim
Nitrofurantoin
Nitroglycerine
Nitric oxide
Isosorbide dinitrate
Amyl nitrate
Nitroprusside
Phenobarbital
|
Quinine sulphate
Primaquine
Chloroquine
Aniline (dyes, ink)
Paraquat
Resorcinol
Chlorate
Benzene derivatives
Naphthalene
Flutamide
Metoclopramide
Phenelzine
|
|
|
|
Lidocaine
Bupivacaine
Mepivacaine
Articaine
Etidocaine
Benzodiazepines
Phenothiazines
|
Propofol
Thiopental
Succinylcholine
Inhalational anaesthetics
Fentanyl
Meperidine
Paracetamol
Aspirin
Phenazopyridine
|
| |
|
At methaemoglobin concentrations below 20%, the patient is usually asymptomatic; however, as levels exceed 20%, blue-grey central cyanosis and chocolate-brown discolouration of the blood begin to manifest. Concentrations above 70% can be fatal (Table 2).4
Table 2 Clinical findings in patients with methaemoglobinaemia4
|
|
|
|
1–3%
|
None
|
|
3–15%
|
Possibly none, low oxygen saturations on pulse oximeter
|
|
15–20%
|
Cyanosis (central and peripheral) not improving with oxygen administration, slate-grey skin colour
|
|
20–50%
|
Dyspnoea, tachypnoea, headache, fatigue, dizziness, syncope, weakness, nausea
|
|
50–70%
|
Metabolic acidosis, dysrhythmia, seizures, central nervous system depression, coma
|
|
>70%
|
Grave hypoxic symptoms, death
|
As demonstrated in this case, ABG analysis can clinch the diagnosis.4 The total FO2Hb, i.e. the proportion of oxygenated haemoglobin in relation to total haemoglobin (including dyshaemoglobins such as methaemoglobin and carboxyhaemoglobin), will be low in these patients. However, it is important to note that they will have normal PaO2 (partial pressure of oxygen in blood) and SaO2. This is because the PaO2 level relates to the fraction of oxygen that is dissolved in blood plasma rather than that bound to haemoglobin.5 As methaemoglobinaemia does not affect oxygen diffusion from the alveoli to the blood plasma, arterial PaO2 will remain normal. SaO2 reflects the level of oxygen bound to haemoglobin but will be falsely normal as its calculation is based on the assumptions of a normal oxygen dissociation curve and physiological levels of dyshaemoglobins, which do not hold true in methaemoglobinaemia.6 However, oxygen saturation measured by pulse oximetry will be reduced because methaemoglobin absorbs the two wavelengths of light that are utilised to non-invasively estimate the percentage of oxyhaemoglobin in the blood.6 Thus, administration of high-flow oxygen will not increase the FO2Hb, SaO2 or resolve blue-grey cyanosis, but it can improve blood gas PaO2 and, to a lesser degree, oxygen saturation on the pulse oximeter.
Patients with methaemoglobin levels of <20% can be managed conservatively by discontinuing the offending agent, as methaemoglobin will be reduced over several hours by the intrinsic activity of methaemoglobin reductase enzymes.4 Patients in respiratory distress should be given high-flow oxygen. Symptomatic patients or those with methaemoglobin levels >20% require treatment with methylene blue infusion (contraindicated in glucose-6-phosphate-dehydrogenase deficiency) which causes non-enzymatic reduction of methaemoglobin. Plasma exchange, haemodialysis, hyperbaric oxygen therapy and supplemental antioxidants (e.g. N-acetylcysteine) can be used as adjuvants or alternative strategies if initial treatment fails.4
In conclusion, methaemoglobinaemia is an uncommon but potentially fatal clinical encounter that is often not considered in patients presenting with hypoxia and cyanosis. It is important that patients on regular oxidising medications (e.g. dapsone) are monitored appropriately for methaemoglobinaemia. Early recognition of the condition and appropriate treatment can result in a favourable outcome for patients.
