An 11-year-old girl presented with bilateral, painless, progressive visual obscuration of one-week duration, without any other focal neuro-deficit. Her history was uneventful. Clinical examination revealed visual acuity of finger counting from 0.6 metres and 1.2 metres from right and left eye, respectively. Fundus examination revealed presence of blurred disc margins without retinal or vessel changes in both eyes. No other focal deficit was ascertainable on physical examination. A clinical diagnosis of bilateral optic neuritis was made. Considering history and examination findings, a demyelinating pathology involving bilateral optic nerves was considered.
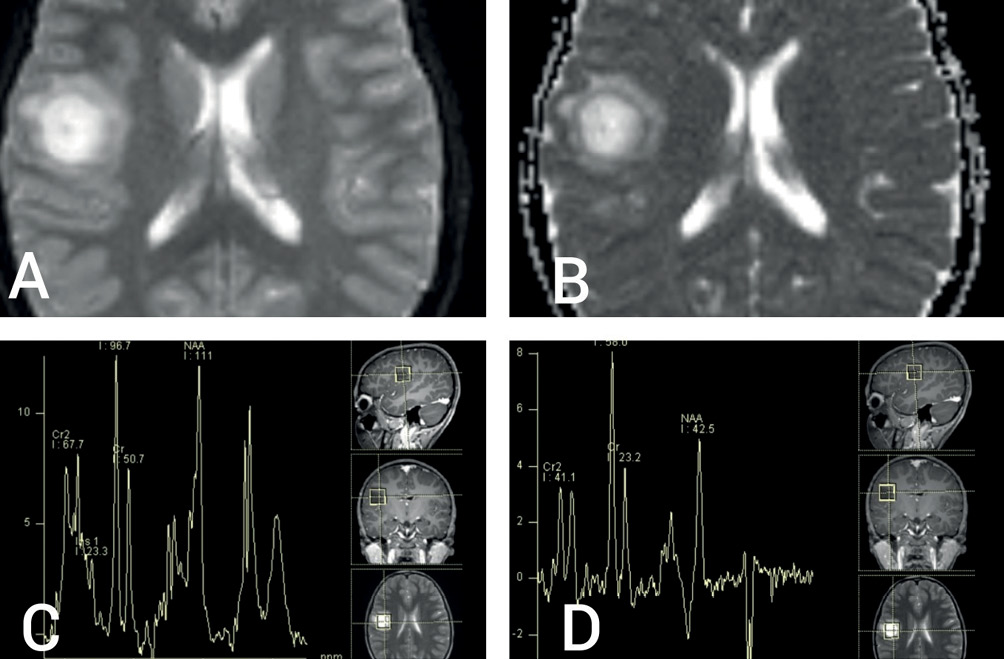
Magnetic resonance imaging (MRI) of brain revealed a round, well-circumscribed lesion in the right parietal lobe, with central hyperintensity and surrounding peri-lesional oedema on the T2-weighted sequence (Figure 1A,B). T1-weighted sequences showed central hypointensity (Figure 1C) with closed ring enhancement on post-contrast images (Figure 1D). Susceptibility-weighted image sequences did not show any evidence of central vein sign. Bilateral optic nerves showed T2 hyperintensity and enhancement on post-contrast T1-weighted images, consistent with clinical impression of optic neuritis. The parenchymal lesion showed high signal on diffusion-weighted (DW) sequence, as well as on the corresponding apparent diffusion coefficient map, not suggestive of true restriction (Figure 2A and 2B). Magnetic resonance spectroscopy showed a high choline peak with low myoinositol levels (Figure 2C and 2D). Magnetic resonance perfusion studies could not be done for logistical reasons. Apart from tumefactive demyelination (TD), differential diagnoses considered were of infectious, metabolic, neoplastic and autoimmune origin.1 A stereotactic biopsy could have been beneficial in this regard; however, it was not consented for by the patient’s kin.
Figure 1 (A) T2-weighted MRI scan showing round, well-circumscribed lesion in right parietal lobe, with central bright hyperintense signal, hypointense rim and mild peri-lesional oedema; (B) T2 fluid attenuated inversion recovery sequence hypointense centre; (C) T1-weighted image showing central hypointensity and (D) T1 post-contrast sequence showing open-ring type of contrast enhancement
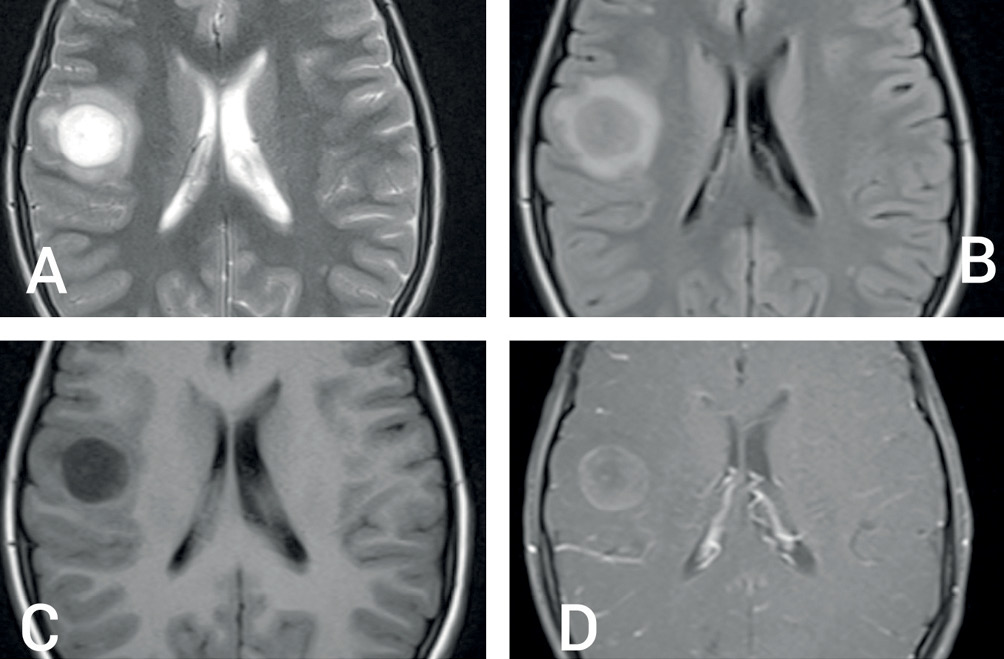
Figure 2 (A) Diffusion-weighted imaging (DWI) MRI sequence showing bright signal without hypointense signal on corresponding apparent diffusion coefficient map (B) suggestive of T2 ‘shine-through’ without true diffusion restriction; (C) and (D) magnetic resonance spectroscopy showing increased choline/creatine ratio and small myoinositol peak without decrease in N-acetyl aspartate levels
Basic laboratory investigations were normal. Vasculitis and infection screen were unremarkable. Anti-aquaporin-4 anti-myelin oligodendrocyte protein antibodies were negative in serum. The cerebrospinal fluid study was normal and oligoclonal bands were absent. A visually evoked potential study revealed bilaterally increased P-100 latencies, suggestive of retino-optic pathway perturbation. The patient was treated with pulse methylprednisolone for three days followed by a tapering dose of oral steroids for two weeks, resulting in prompt clinical improvement. Three-month follow-up was uneventful. Repeat MRI scans of the brain revealed resolution of the original lesion without additional lesions.
Tumefactive demyelination are tumour-like, demyelinating lesions larger than 2 cm, commonly situated in the fronto-parietal lobes.2,3 Closest differentials include abscess and neoplasm. Prominent surrounding oedema, which may be seen in acute cases, is less suggestive of TD compared to a neoplasm.2,3 The presence of open-ring type of contrast enhancement, with the open edge directed towards the cortex or basal ganglia, is highly suggestive of demyelination, with specificities ranging from 84% to 94%, as evaluated by Masdeu et al.4 Interestingly, hypoattenuation of the corresponding MRI-enhanced area on a computed tomography scan is another radiological clue.5 Lack of diffusion restriction on MRI DW scans are helpful in making the distinction between an abscess and a neoplasm.6 Despite its ominous radiological appearance, TD exhibits a monophasic course in one-third of cases, with progression to a relapsing-remitting pattern of multiple sclerosis in the remaining cases. However, progressive neurological decline from disease onset is rare.2,3
Stereotactic biopsy may not always be a feasible option when it comes to evaluation of suspected TD. Disease course and clinico-radiological correlation are paramount in determining the nature of the pathology. 
