Carbapenem-resistant Enterobacteriaceae (CRE) has become a major threat to public health. It is increasingly reported worldwide, including in Asian countries.1 CRE is listed by the World Health Organization (WHO) as an antibiotic-resistant critical-priority pathogen.2 From a recent meta-analysis, infections with CRE result in mortality rates two to three times higher than those for infections caused by carbapenem-sensitive Enterobacteriaceae.3 The 30-day mortality rate among patients with CRE infections is high (63.8%) compared with other multidrug-resistant organism (MDRO) infections.4
Sungai Buloh Hospital is a renowned tertiary care hospital and a centre of referral, mainly for infectious disease, orthopaedic, trauma and neurosurgical cases. In 2016 we noticed a sudden spike of unrelated and unlinked CRE-infected and colonised patients. Since then, the hospital has stepped up its contact tracing and screening strategy. CRE has the potential for widespread transmission of resistance via mobile genetic elements. Hence, infection prevention and control (IPC) is the cornerstone of CRE management.
Interventions such as surveillance, isolation precautions and antimicrobial stewardship (AMS) play a vital role in controlling further spread of CRE. Understanding more about the risk factors will help in implementing a targeted screening strategy to identify patients at risk early. It has been well-recognised that increasing antibiotic consumption is a major risk factor for acquiring CRE.5 Some other reported risk factors include length of hospital stay, the presence of invasive devices and co-colonisation by other MDROs.6–8
Management of CRE is a major challenge for clinicians because of the limited treatment options and poor outcomes. For these reasons, baseline surveillance to determine the CRE risk factors in an institution is the key to successful interventions.9 The aim of this study is to identify the risk factors associated with CRE infection and colonisation in a tertiary hospital in Malaysia.
Methods
Study design and population
The study included data collected from 1 January 2017 to 31 December 2019 in Sungai Buloh Hospital. During that time, whenever a patient was identified as having a CRE infection or colonisation, all close contacts were immediately identified and rectal screening was done for CRE carriage. If the first screening was negative, another screening was done after seven days, provided the patients were still hospitalised.
This is a retrospective case-control study with a ratio of 1:1. Cases were defined as patients with CRE infection or colonisation admitted to adult wards. The CRE samples could be isolated from any source. For each patient with CRE, one control was randomly selected from patients who had undergone contact screening and who had a negative CRE rectal swab. The case and control patients were matched for the time of sampling and ward location. If a control was not available, the CRE patient case was excluded. Other exclusion criteria include outpatient CRE samples and patients who were discharged against medical advice.
Data collection
The CRE and contact screening records were obtained from the infection control unit. Subjects with CRE isolated from multiple sites or on multiple dates were counted only once and information from the first event was taken. Each patient case was randomly paired with a control as defined above. Data was extracted from the hospital’s electronic medical records and filled into case report forms. The characteristics and risk factors were collected as below:
- Demographics (age, gender, ethnic group)
- Comorbidities (diabetes mellitus, immunodeficiency, chronic diseases of lung, cardiovascular, kidney and liver)
- History of admission to hospital or intensive care unit (ICU) for the past one year
- Length of stay prior to the positive CRE result (index admission)
- Unable to ambulate on admission (at least wheelchair bound)
- Invasive procedures, surgical procedures, indwelling devices, presence of other MDROs and exposure to antimicrobial therapy (≥48 hours) for the past six months
- Total length of stay
- In-hospital mortality rate
Microbiological method
Carbapenem susceptibility was first tested using a disk diffusion method. Non-susceptibility was confirmed by using the ETEST® system (bioMérieux) and the modified carbapenem inactivation method (mCIM). The tests were interpreted in accordance with the 2015 Clinical and Laboratory Standards Institute guidelines. Isolates were considered resistant to carbapenems when they were intermediate susceptible or resistant to at least one of the carbapenems tested.
Statistical analysis
Continuous variables were expressed as means and standard deviations unless otherwise stated. Categorical variables were described using frequencies and percentiles. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test, whereas non-categorical variables were tested using a student t-test or Mann–Whitney U test. Multivariate analysis was performed for variables with P<0.1 in univariate analysis. Independent risk factors of CRE acquisition were evaluated using logistic regression. A P value of less than 0.05 is considered statistically significant. Statistical analysis was done using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
Ethical approval
The study was approved by the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia.
Results
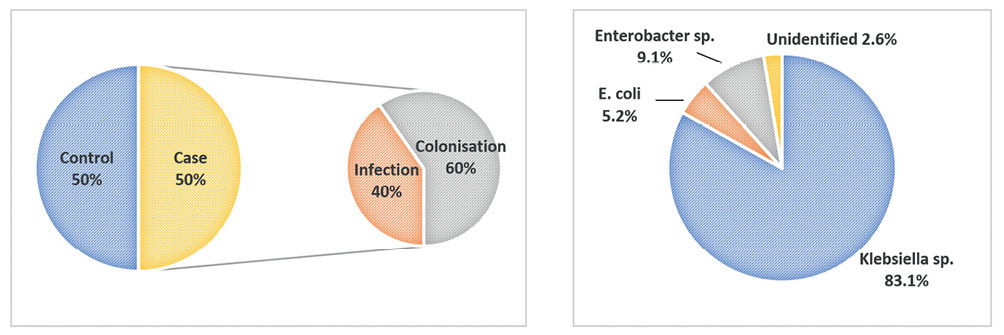
A total of 154 subjects were included in the study. There were 77 case patients and 77 control patients. Among the cases, there were 31 patients (40%) with CRE infection and 46 patients (60%) with CRE colonisation (Figure 1). The baseline characteristics of both groups appeared to be balanced (Table 1). The mean ages for case patients and control patients were 56 (SD±18.4) and 53 (SD±18.8) respectively. There were more male patients in the study, but no difference was observed between case and control groups (65% and 64% respectively, P=0.866). Different ethnicities were well-represented in this study. The majority were Malay (62%), followed by Chinese (21%) and Indian (14%).
Figure 1 Left: Percentage of cases (infection and colonisation) and controls. Right: Percentage of isolated CRE organisms
Diabetes mellitus (31%), ischaemic heart disease (20%) and chronic kidney disease (CKD) or end-stage renal failure (ESRF) (14%) were among the common comorbidities in all patients in the study, but none of them were statistically significant. However, patients who were unable to ambulate on admission showed higher significance of acquiring CRE (OR: 2.636; 95% CI: 1.339–5.192). For the purpose of analysis, the risk factors of exposure were divided into exposures during the current admission, the past six months and the past one year, as shown in Table 2. There were more CRE case patients with a history of hospitalisation, ICU admission and mechanical ventilation (including during the past one year and current admission). However, none of them showed significant differences statistically.
Table 1 Demographic and comorbidities of case and control
| |
|
|
|
|
|
Age (mean ± SD, years)
|
56 ± 18.4
|
53 ± 18.8
|
1.010[0.992–1.027]
|
0.275a
|
|
Gender, male
|
50 (65%)
|
49 (64%)
|
1.058[0.547–2.046]
|
0.866
|
|
Ethnicity
|
|
|
|
|
|
Malay
|
47 (61%)
|
49 (64%)
|
|
|
|
Chinese
|
17 (22%)
|
16 (21%)
|
|
|
|
Indian
|
9 (12%)
|
12 (16%)
|
|
|
|
Other
|
4 (5%)
|
0
|
|
|
| |
|
|
|
|
|
Comorbidities
|
|
|
|
|
|
Diabetes mellitus
|
28 (36%)
|
20 (26%)
|
1.629[0.817–3.244]
|
0.165
|
|
CKD/ESRD
|
13 (17%)
|
9 (12%)
|
1.535[0.614–3.835]
|
0.359
|
|
Ischaemic heart disease
|
13 (17%)
|
18 (23%)
|
0.666[0.300–1.476]
|
0.317
|
|
Chronic lung disease
|
2 (3%)
|
4 (5%)
|
0.487[0.086–2.739]
|
0.681
|
|
Chronic obstructive airway disease
|
4 (5%)
|
1 (1%)
|
4.164[0.455–38.141]
|
0.367
|
|
Immunodeficiency
|
3 (4%)
|
6 (8%)
|
0.480[0.116–1.992]
|
0.495
|
|
Chronic liver disease
|
3 (4%)
|
4 (5%)
|
0.740[0.160–3.422]
|
0.699
|
|
Unable to ambulate on admission
|
37 (48%)
|
20 (26%)
|
2.636[1.339–5.192]
|
0.005
|
Table 2 Risk factors and outcome of case and control patients
| |
|
|
|
|
|
Exposure within past one year
|
|
|
|
|
|
Hospitalisation
|
32 (42%)
|
24 (31%)
|
1.570[0.810–3.044]
|
0.180
|
|
ICU admission
|
9 (12%)
|
2 (3%)
|
4.963[1.036–23.738]
|
0.056
|
|
Mechanical ventilation
|
10 (13%)
|
5 (6%)
|
2.149[0.699–6.613]
|
0.174
|
| |
|
|
|
|
|
Exposure in current admission
|
|
|
|
|
|
ICU admission
|
34 (44%)
|
28 (36%)
|
1.384[0.725–2.641]
|
0.324
|
|
Mechanical ventilation
|
39 (51%)
|
30 (39%)
|
1.608[0.848–3.049]
|
0.145
|
|
|
|
|
|
|
|
Exposure within past six months
|
|
|
|
|
|
Invasive procedure (endoscopy, bronchoscopy, angiogram)
|
22 (29%)
|
17 (22%)
|
1.412[0.680–2.933]
|
0.354
|
|
Surgery
|
35 (42%)
|
30 (39%)
|
1.306[0.688–2.479]
|
0.415
|
|
Indwelling devices (all)
|
60 (78%)
|
43 (56%)
|
2.791[1.384–5.629]
|
0.004
|
|
Central venous line
|
43 (56%)
|
29 (38%)
|
2.093[1.099–3.986]
|
0.024
|
|
Endotracheal tube
|
39 (51%)
|
32 (42%)
|
1.443[0.764–2.727]
|
0.258
|
|
Drain
|
38 (49%)
|
35 (27%)
|
1.804[0.945–3.446]
|
0.073
|
| |
|
|
|
|
|
Exposure within past six months
|
|
|
|
|
|
Concomitant other MDRO (all)
|
23 (30%)
|
11 (14%)
|
2.556[1.144–5.707]
|
0.020
|
|
ESBL
|
13 (17%)
|
6 (8%)
|
2.404[0.863–6.697]
|
0.086
|
|
MRO
|
11 (14%)
|
2 (3%)
|
6.250[1.337–29.227]
|
0.017
|
|
MRSA
|
11 (14%)
|
3 (4%)
|
4.111[1.099–15.375]
|
0.046
|
| |
|
|
|
|
|
Exposure within past six months
|
|
|
|
|
|
Antibiotic (all)
|
70 (91%)
|
55 (71%)
|
4.000[1.593–10.047]
|
0.002
|
|
Carbapenem
|
26 (34%)
|
11 (14%)
|
3.059[1.383–6.767]
|
0.005
|
|
Cephalosporin
|
50 (65%)
|
39 (51%)
|
1.804[0.945–3.446]
|
0.073
|
|
BLBLI and penicillin
|
45 (58%)
|
32 (42%)
|
1.978[1.042–3.754]
|
0.036
|
|
Piperacillin-tazobactam
|
27 (35%)
|
14 (18%)
|
2.430[1.154–5.117]
|
0.018
|
|
Vancomycin
|
12 (16%)
|
4 (5%)
|
3.369[1.035–10.964]
|
0.035
|
|
Fluoroquinolone
|
4 (5%)
|
2 (3%)
|
2.055 [0.365–11.564]
|
0.405
|
| |
|
|
|
|
|
Length of stay prior to positive CRE (median days, IQR)
|
17 (24)
|
12 (14)
|
Z statistic −2.200a
|
0.028
|
|
≥2 weeks
|
45 (58%)
|
35 (45%)
|
1.688[0.892–3.193]
|
0.107
|
|
≥3 weeks
|
32 (42%)
|
18 (23%)
|
2.331[1.163–4.673]
|
0.016
|
|
Total length of stay (median days, IQR)
|
35 (56)
|
19 (32)
|
Z statistic −3.502a
|
<0.001
|
|
In-hospital mortality
|
30 (39%)
|
19 (25%)
|
1.948[0.976–3.891]
|
0.059
|
For exposures within the past six months, CRE case patients had a significantly higher proportion of indwelling devices (OR: 2.791; 95% CI: 1.384–5.629), concomitant with other MDROs (OR: 2.556; 95% CI: 1.144–5.707) and antibiotic exposure (OR: 4.000; 95% CI: 1.593–10.047). The subanalysis in Table 2 shows that central venous line (CVL) (OR: 2.093; 95% CI: 1.099–3.986), multi-resistant organism (MRO) (OR: 6.250; 95% CI: 1.337–29.227) and methicillin-resistant Staphylococcus aureus (MRSA) (OR: 4.111; 95% CI: 1.099–15.375) were associated with CRE infection or colonisation.
Almost all antibiotics showed significant association with CRE cases. These include carbapenem, penicillin and β-lactam/β-lactamase inhibitor (BLBLI), piperacillin-tazobactam and vancomycin. In view of the skewed distribution, the length of stay was expressed as median days and analysed using a Mann–Whitney U test. CRE case patients had longer hospitalisation prior to a positive CRE result (median 17 days; IQR 24 days) compared with control patients (median 12 days; IQR 14 days; P=0.028). When analysed by weeks of hospitalisation, three weeks or longer was the significant cut-off (OR: 2.331; 95% CI: 1.163–4.673).
Multivariate logistic regression analysis (Table 3) revealed that being unable to ambulate on admission (adjusted OR: 2.345; 95% CI: 1.170–4.699) and having prior antibiotic exposure (adjusted OR: 3.515; 95% CI: 1.377–8.972) were independent risk factors in acquiring CRE. CRE infection and colonisation resulted in prolonged hospitalisation (median 35 days; IQR 56 days; P<0.001). There was no difference in hospital mortality among subjects in the case and control groups. In subgroup analysis (Figure 2), subjects with CRE infection had a higher in-hospital mortality rate (64.5%) compared with control patients (P<0.001). The non-bacteraemia group had an equally high mortality rate (60%) compared with the bacteraemia group (68.8%), and no difference was observed (P=0.61).
Table 3 Multivariate analysis of risk factors associated with CRE
|
|
|
|
|
Unable to ambulate
on admission
|
2.345
[1.170–4.699]
|
0.016
|
|
Antibiotic exposure within past six months
|
3.515
[1.377–8.972]
|
0.009
|
Discussion
The first report of carbapenem-resistant K. pneumoniae in Malaysia was an imipenem-resistant strain isolated from the blood culture of a 42-year-old female in 2004.10 In Asia, the prevalence of CRE was still low during the earlier study period (2000–2012) with average resistance rates of 0.6% to imipenem and 0.9% to meropenem. However, the resistance rates to imipenem and meropenem in Enterobacteriaceae exhibited a steadily escalating trend.1 The prevalence of CRE in Malaysia varied between 0.3% and 5.74% across different centres.11–13 The majority of the carbapenemase were the New Delhi metallo-β-lactamase 1 (NDM-1) and OXA-48 genes.12–14 The high diversity of carbapenem resistance genes in Malaysia was attributed to the presence of plasmid-localised blaNDM (blaNDM-1/blaNDM-5) or blaKPC (blaKPC-2/blaKPC-6).15 Most (85.5%) of the isolated CRE in Malaysia was reported as Klebsiella species.13 This was similar to the finding in our study of 83%.
From our case-control study, most of the CRE risk factors were consistent with other published literature. These include exposure to antibiotics, the presence of a CVL, co-colonisation with other MDROs and prolonged hospitalisation.6–8,16–18 Interestingly, our multivariate analysis showed that not being able to ambulate on admission was an independent risk factor for acquiring CRE. This was also reported by authors from Singapore and Japan.6,18 In long-term care facilities (LTCF) in Italy,19 residents with physical disabilities were associated with MDRO colonisation. This was similarly found among nursing home residents in Michigan with functional disability.20
Meta-analysis has shown that overuse of carbapenem is the main antibiotic contributing to the emergence of CRE.21 Apart from carbapenem, our study found that exposure to piperacillin-tazobactam, BLBLI and penicillin was also associated with CRE infection and colonisation. These associations were similarly found in Korea and Singapore for exposure to penicillin and BLBLI,6,8 and in Greece for exposure to antipseudomonal penicillin.16 It is estimated that 20–50% of all antibiotics prescribed in acute care hospitals in the USA are either unnecessary or inappropriate.22 Therefore, an antibiotic stewardship programme is an important intervention to reduce CRE23 by emphasising appropriate indication, choice and duration of antimicrobial therapy.
Prolonged hospitalisation is a well-known sequela for complicated CRE infection. Our analysis looking specifically at duration of hospitalisation prior to positive CRE isolation provided additional evidence to stratify patients at risk. Patients who were hospitalised for more than three weeks (or median 17 days, IQR 24 days) were found to be at risk of CRE acquisition. This could be a key criterion in refining a screening strategy. A recent study on the impact of various MDROs on hospitalisation supported this finding,24 where longer hospital stays prior to the onset of infection (mean 16.3 ± 14.1 days) were associated with hospital-acquired infection.
In a meta-analysis in 2018, the CRE infection mortality rate ranged from 18.6–94.1%. Compared with carbapenem-sensitive Enterobacteriaceae (CSE), CRE was associated with a significantly higher risk of overall mortality (OR: 3.39; 95% CI: 2.35–4.89).3 Our study demonstrated a high CRE infection mortality rate (64.5%) despite treatment in most if not all patients. Non-bacteraemia CRE infection should be given the same attention due to its high mortality at 60% (no difference compared with the bacteraemia group, 68.8%, P=0.61). Another consequence of CRE infection is the high cost of treatment. In Japan, medical expenses for admission in the CRE group were reported to be about three times higher than those in the CSE group.18
A significant proportion of colonised patients may develop CRE infection later. In a recent meta-analysis, colonised patients had a 16.5% cumulative infection rate.25 In another study based in an ICU setting, the infection rate increased dramatically with almost half (47%) of the colonised patients developing CRE infection within 30 days, representing a tenfold increase in the odds of infection compared with non-colonised patients. Notably, the colonising and infecting organisms were the same species in all but one patient.26 Even though universal screening for CRE may be easier to implement, it is costly and a considerable workload for the microbiological laboratory. A modified strategy such as having an active surveillance culture based on population at risk has been reported to significantly reduce nosocomial transmission of CRE.27
The principal limitation of our study was the retrospective design. Data from the medical records was insufficient to further categorise physical disability based on scoring systems such as the Barthel immobility score28 or the Arling scale.29 In addition, due to the control group matching criteria, the differences in ward locality and subspecialty were not investigated. A small number of subjects (n=4) were excluded because the control arm was unavailable, they were discharged against medical advice or they were in the paediatric age group.
In conclusion, in this study, CRE infection resulted in high mortality and prolonged hospitalisation. The risk factors associated with CRE infection and colonisation were a history of antibiotic exposure, presence of an indwelling device, co-colonisation with other MDROs, prolonged hospitalisation of more than three weeks and being unable to ambulate on admission. Our study provided a first insight into the epidemiology and risk factors of CRE in a tertiary hospital in Malaysia. These findings will refine the criteria of a targeted screening and hence help control the spread of CRE. 
Acknowledgements
The authors would like to thank Tan Sri Dr Noor Hisham Abdullah (Director General of Health, Malaysia), Dato’ Indera Dr Sha’ari Bin Ngadiman (State Health Director of Selangor, Malaysia) and Dr Kuldip Kaur A/P Prem Singh (Director of Sungai Buloh Hospital, Selangor, Malaysia) for the permission to publish this article. The authors also thank the following for their contribution to this study: Yan Chyi Tan, Nur Aida Syuhadha Hakimin and Muhamad Afiq Mu’iz Arifin from Sungai Buloh Hospital, Selangor, Malaysia.
References
1 Xu Y, Gu B, Huang M et al. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000–2012 in Asia. J Thorac Dis 2015; 7: 376–85.
2 Tacconelli E, Carrara E, Savoldi A et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318–27.
3 Martin A, Fahrbach K, Zhao Q et al. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis 2018; 5: ofy150-ofy.
4 Sabino S, Soares S, Ramos F et al. A cohort study of the impact of carbapenem-resistant Enterobacteriaceae infections on mortality of patients presenting with sepsis. mSphere 2019; 4: e00052-19.
5 McLaughlin M, Advincula MR, Malczynski M et al. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob Agents Chemother 2013; 57: 5131–3.
6 Ling ML, Tee YM, Tan SG et al. Risk factors for acquisition of carbapenem resistant Enterobacteriaceae in an acute tertiary care hospital in Singapore. Antimicrob Resist Infect Control 2015; 4: 26.
7 Segagni Lusignani L, Presterl E, Zatorska B et al. Infection control and risk factors for acquisition of carbapenemase-producing enterobacteriaceae. A 5 year (2011–2016) case-control study. Antimicrob Resist Infect Control 2020; 9: 18.
8 Kang JS, Yi J, Ko MK et al. Prevalence and risk factors of carbapenem-resistant Enterobacteriaceae acquisition in an emergency intensive care unit in a tertiary hospital in Korea: a case-control study. J Korean Med Sci 2019; 34: e140.
9 Richter SS, Marchaim D. Screening for carbapenem-resistant Enterobacteriaceae: who, when, and how? Virulence 2017; 8: 417–26.
10 Palasubramaniam S, Karunakaran R, Gin GG et al. Imipenem-resistance in Klebsiella pneumoniae in Malaysia due to loss of OmpK36 outer membrane protein coupled with AmpC hyperproduction. Int J Infect Dis 2007; 11: 472–4.
11 Zainol Abidin NZ, Sulong A, Alfizah H et al. Molecular detection of the New Delhi metallo-β-lactamase-1 gene in Enterobacteriaceae isolates in a tertiary medical centre. Malays J Pathol 2015; 37: 227–32.
12 Mohamed N, Said MH, Hussin H et al. Carbapenem-resistant Enterobactericeae: clinico-epidemiological perspective. Trop Biomed 2018; 35: 300–7.
13 Zaidah AR, Mohammad NI, Suraiya S et al. High burden of carbapenem-resistant Enterobacteriaceae (CRE) fecal carriage at a teaching hospital: cost-effectiveness of screening in low-resource setting. Antimicrob Resist Infect Control 2017; 6: 42.
14 Low YM, Yap PS, Abdul Jabar K et al. The emergence of carbapenem resistant Klebsiella pneumoniae in Malaysia: correlation between microbiological trends with host characteristics and clinical factors. Antimicrob Resist Infect Control 2017; 6: 5.
15 Gan HM, Eng WWH, Dhanoa A. First genomic insights into carbapenem-resistant Klebsiella pneumoniae from Malaysia. J Glob Antimicrob Resist 2020; 20: 153–9.
16 Falagas ME, Rafailidis PI, Kofteridis D et al. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case control study. J Antimicrob Chemother 2007; 60: 1124–30.
17 Nicolas-Chanoine MH, Vigan M, Laouenan C et al. Risk factors for carbapenem-resistant Enterobacteriaceae infections: a French case-control-control study. Eur J Clin Microbiol Infect Dis 2019; 38: 383–93.
18 Asai N, Sakanashi D, Suematsu H et al. The epidemiology and risk factor of carbapenem-resistant enterobacteriaceae colonization and infections: case control study in a single institute in Japan. J Infect Chemother 2018; 24: 505–9.
19 Nucleo E, Caltagirone M, Marchetti VM et al. Colonization of long-term care facility residents in three Italian provinces by multidrug-resistant bacteria. Antimicrob Resist Infect Control 2018; 7: 33.
20 Min L, Galecki A, Mody L. Functional disability and nursing resource use are predictive of antimicrobial resistance in nursing homes. J Am Geriatr Soc 2015; 63: 659–66.
21 van Loon K, Voor in ’t holt AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62: e01730-17.
22 Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 2014; 59 Suppl 3: S97–100.
23 Viale P, Tumietto F, Giannella M et al. Impact of a hospital-wide multifaceted programme for reducing carbapenem-resistant Enterobacteriaceae infections in a large teaching hospital in northern Italy. Clin Microbiol Infect 2015; 21: 242–7.
24 Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés Pet al. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis 2017; 65: 644–52.
25 Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: A systematic review. Am J Infect Control 2016; 44: 539–43.
26 McConville TH, Sullivan SB, Gomez-Simmonds A et al. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS One 2017; 12: e0186195.
27 Ben-David D, Maor Y, Keller N et al. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect Control Hosp Epidemiol 2010; 31: 620–6.
28 Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965; 14: 61–5.
29 Arling G, Nordquist RH, Brant BA, Capitman JA. Nursing home case mix. Patient classification by nursing resource use. Med Care 1987; 25: 9–19.
