Emphysematous pyelonephritis (EPN) is an uncommon, acute, severe form of necrotising infection affecting the renal parenchyma, collecting system and surrounding tissues. The hallmark feature is an accumulation of gas within these structures.1–3 Risk factors for EPN include diabetes mellitus (DM), obstructive uropathy, vesicoureteric reflux and immunosuppression, although patients without DM are not immune against EPN and EPN may be a complication in patients with renal papillary necrosis.1–5 Patients may present with fever, loin pain, vomiting, altered consciousness and shock.1-3
Various imaging modalities can identify gas in the affected renoureteral units. Abdominal X-ray may suggest the presence of gas within the renal outlines and prompt a computed tomography (CT) scan. Ultrasonography may reveal hypoechoic kidneys with echogenic components and dirty shadows in the renal parenchyma, calyces and pelvis. However, the CT scan is most reliable for establishing the diagnosis of EPN and classification: class 1, gas in the collecting system only; class 2, gas in the renal parenchyma without extension to the extrarenal space; class 3A, extension of gas or abscess to the perinephric space; class 3B, extension of gas or abscess to the pararenal space; class 4, bilateral EPN or a solitary kidney with EPN.1,3
Cases of EPN are rare, and patients are managed differently in different centres, although some treatment protocols and management algorithms have been proposed by different researchers and investigators.1,3,7 The principles of treatment include resuscitation, intravenous antibiotics, glycaemic control (in diabetics), interventions aiming to release obstructions, and nephrectomy (in selected cases).1,3
Outcomes for patients with EPN are variable and depend on status at presentation, radiological class and quality of care.1,2
In this report, we describe the clinical characteristics, laboratory and imaging findings and in-hospital outcomes of patients diagnosed with EPN.
Methods
This retrospective observational study included 20 patients diagnosed with EPN who were managed in the Department of Internal Medicine and Department of Nephrology at the Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM) General Hospital in Dhaka, Bangladesh between August 2014 and February 2020. BIRDEM General Hospital is one of the world’s largest hospitals dedicated to diabetic patients, but patients without DM are also treated here. It has 725 inpatient beds and there are about 3000 outpatient visits every day.
The participants were evaluated by a team consisting of physicians and/or nephrologists, radiologists and urologists after undergoing a CT scan to determine whether urologic intervention was required. Case records were completed during discharge (or would be after death, but none occurred) and contained selected sociodemographic, clinical, laboratory and imaging characteristics, and in-hospital treatment outcomes. Exclusions included the presence of a fistula between the genitourinary tract and gut and undergoing a recent genitourinary procedure.
The study protocol was approved by the Institutional Review Board of BIRDEM General Hospital (BIRDEM/IRB/2020/221) and informed consent was taken from all patients before enrolment or discharge from hospital. Statistical Package for Social Scientists (SPSS version 20) was used to analyse the data, comparing different demographic, clinical and laboratory parameters and CT classes among patients treated with or without nephrectomy. A p value of < 0.05 was taken as significant.
Results
A total of 20 patients were recruited to the study (mean age 49.4 ± 12.3 years; range 24–70) including 14 females (70%). All of the patients were diabetic (one was a new case). Other common comorbidities were hypertension (10 patients; 50%), chronic kidney disease (4 patients; 20%), enlarged prostate (3 patients; 15%) and renal stones (2 patients; 10%). Six patients were initially treated in other hospitals. The common clinical features were fever (100%), loin pain and/or renal-angle tenderness (18 patients; 90%), vomiting (17 patients; 85%), dysuria (9 patients; 45%), increased urinary frequency (4 patients; 20%) and dehydration (6 patients; 30%). One patient (5%) presented with altered sensorium. The patients had experienced these symptoms for 3–14 days before presenting at our hospital.
Neutrophilic leucocytosis was common and four patients had thrombocytopaenia. All had high erythrocyte sedimentation rates (ESR) and C-reactive protein (CRP) levels. Overall, glycaemic status was poor (Table 1). Other features were pyuria (17 patients; 85%), glycosuria (19 patients; 95%) and microscopic haematuria (7 patients; 35%). Fourteen cases (70%) were complicated by acute kidney injury (AKI),8 mostly at stage 1 (11/14; 78.6%) and 11 (55%) had hyponatraemia. The laboratory characteristics are shown in Table 1.
Table 1 Laboratory parameters of patients with emphysematous pyelonephritis (n = 20)
|
Parameter
|
Values and number of patients
(mean ± standard deviation)
|
Reference value
|
|
Haemoglobin (mean ± standard deviation; range)
|
10.27 ± 1.92; 7.0–15.1
|
11.5–16.5 g/dL
|
|
Total white cell count (mean ± standard deviation; range)
|
22.46 ± 7.52; 10.25–36.63
|
4–11.0 x 109/L
|
|
Leukocytosis (> 11.0 x 109/L)
|
19 (95%)
|
—
|
|
Thrombocytopaenia (< 150.0 x 109/L)
|
4 (20%)
|
150–450 x 109/L
|
|
Random blood glucose at admission (mean ± standard deviation; range)
|
17.83 ± 5.93; 11.1–35.5
|
4.4–7.8 mmol/L
|
|
Glycated haemoglobin (HbA1c) (mean ± standard deviation; range)
|
84.35 ± 20.85; 52–146
|
20–42 mmol/mol
|
|
Blood urea (mean ± standard deviation; range)
|
11.11 ± 6.80; 3.66–31.30
|
2.5–6.6 mmol/L
|
|
Serum creatinine (mean ± standard deviation; range)
|
220.51 ± 124.21; 44.20–459.69
|
64–111 [greek mu]mol/L
|
|
Acute kidney injury (AKI)8
|
14 (70%)
|
—
|
|
Stage 1
|
11/14 (78.6%)
|
—
|
|
Stage 2
|
3/14 (21.4%)
|
—
|
|
Hyponatraemia (< 135 mmol/L)
|
11 (55%)
|
135–146 mmol/L
|
|
Hypokalaemia (< 3.5 mmol/L)
|
3 (15%)
|
3.5–5.2 mmol/L
|
|
Hypoalbuminaemia (< 34 g/L)
|
12 (60%)
|
34–50 g/L
|
The diagnosis of EPN was confirmed by abdominal CT scan. One patient had EPN in an ectopic right kidney and one had EPN with a psoas abscess. The right kidney was involved in 9 patients (45%) and the left in 11 patients (55%). Two had evidence of pyelonephritis in contralateral kidneys along with EPN.
According to the Huang and Tseng classification,3 three patients (15%) had class 3B EPN (Figure 1), one (5%) had class 3A, and 16 (80%) had class 2 (Figure 2).
Figure 1 Non-contrast CT scan of the abdomen (axial film) showing class 3B (gas and abscess extending beyond renal fascia) emphysematous pyelonephritis of the left kidney (wide arrow) with bilateral renal stones (narrow arrows)
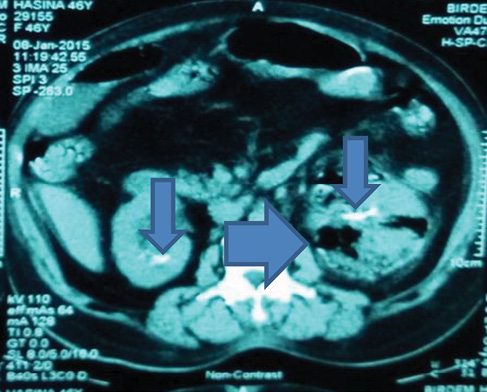
Figure 2 Non-contrast CT scan of the abdomen (axial film) showing class 2 (gas within renal parenchyma) emphysematous pyelonephritis of the right kidney (wide arrow) with gross parenchymal destruction
Urine culture revealed the growth of microorganisms in 16 patients (80%). Escherichia coli was the most common organism identified on urine culture (12; 60%), of whom five (41.7%) had extended-spectrum beta-lactamase (ESBL)-positive E. coli; three of these cases (25%) were complicated by bacteraemia. Blood appeared to be sterile in all other cases. Less common microorganisms were Klebsiella pneumoniae (1 patient; 5%), Candida albicans (1; 5%), non-albicans Candida (1; 5%) and mixed bacterial species (1; 5%). One patient had sterile urine and blood, but culture of pus collected during nephrectomy revealed growth of ESBL-positive E. coli.
All patients were treated with intravenous antibiotics and other supportive measures including fluid resuscitation. Two patients underwent a few sessions of haemodialysis. Four (20%) required surgical interventions (nephrectomy in 3; 15%) and open drainage (1; 5%). The requirement for nephrectomy was associated with a higher radiological class of EPN (p = 0.034) and AKI (p = 0.032) (Table 2). No patient required percutaneous drainage or double-J (DJ) stenting. Biopsies of nephrectomised tissues revealed evidence of acute-on-chronic nephritis with micro-abscess formation. The patients were hospitalised for 7–31 days. AKI resolved in 8 patients before discharge and there were no deaths. The management and outcomes are shown in Table 3.
Table 2 Comparison of different parameters among emphysematous pyelonephritis patients treated with (n = 3) and without nephrectomy (n = 17)
|
Parameter
|
EPN patients treated with nephrectomy
(n = 3)
|
EPN patients treated without nephrectomy
(n = 17)
|
p value
|
|
Mean age (years)
|
49.3 ± 3.1
|
49.4 ± 13.8
|
0.998
|
|
Sex (F/M)
|
3/0
|
11/6
|
0.30
|
|
Renal stones (n = 2)
|
Present
|
1 (33.34%)
|
1 (5.88%)
|
0.932
|
|
Absent
|
2 (66.66%)
|
16 (94.12%)
|
|
Haemoglobin (g/dL)
|
10.46 ± 0.46
|
10.24 ± 2.14
|
0.541
|
|
Thrombocytopaenia
(n = 4)
|
Present
|
1 (33.34%)
|
3 (17.65%)
|
0.071
|
|
Absent
|
2 (66.66%)
|
14 (82.35%)
|
|
Hypoalbuminaemia
(n = 10)
|
Present
|
2 (66.66%)
|
8 (47.05%)
|
0.64
|
|
Absent
|
1 (33.34%)
|
9 (52.95%)
|
|
Acute kidney injury (AKI)
(n = 14)
|
Present
|
2 (66.66%)
|
12 (70.58%)
|
0.032
|
|
Absent
|
1 (33.34%)
|
5 (29.42%)
|
|
Bacteraemia (n = 3)
|
Present
|
1 (33.34%)
|
2 (11.76%)
|
0.733
|
|
Absent
|
2 (66.66%)
|
15 (88.24%)
|
|
Emphysematous pyelonephritis (EPN) class
|
Class 2
|
1 (33.34%)
|
15 (88.24%)
|
0.034
|
|
Class 3B (n = 3)
Class 3A (n = 1)
|
2 (50.00%)
—
|
1 (25%)
1 (25%)
|
|
Mean hospital stay (days)
|
21.3 ± 6.7
|
14.7 ± 5.5
|
0.086
|
Table 3 Management and outcome of patients with emphysematous pyelonephritis (n = 20)
|
Treatment/outcome
|
Frequency (%)
|
|
Antibiotic
|
20 (100)
|
|
Meropenem
|
14 (70)
|
|
Ceftazidime
|
3 (15)
|
|
Ceftriaxone
|
2 (10)
|
|
Piperacillin and tazobactum (combination)
|
1 (5)
|
|
Acute kidney injury (AKI)
|
14 (70)
|
|
Resolved before discharge
|
8 (57.1)
|
|
Improving/resolving at discharge
|
6 (42.9)
|
|
Haemodialysis required
|
2 (10)
|
|
Surgical intervention required
|
4 (20)
|
|
Nephrectomy
|
3 (15)
|
|
Open drainage
|
1 (5)
|
|
Outcome
|
|
|
Survival
|
20 (100)
|
|
Death
|
0
|
Discussion
In 1898, Kelly and MacCallum were the first to report a case of gas-forming renal infection (pneumaturia).9 Since then, various terms like ‘pneumonephritis’ and ‘renal emphysema’ have been used to describe this entity. The term ‘emphysematous pyelonephritis’ was adopted by Schultz and Klorfein in 1962.2,10
EPN predominantly occurs in females, and most commonly involves the left kidney, followed by the right kidney; bilateral involvement is the least common.3,7,11 There are exceptional cases of bilateral renal involvement (class 4) and in which concomitant emphysematous cystitis has been reported.12No patients in our series had bilateral (class 4) disease, in contrast to the study of Misgar et al. in which a third of their EPN patients had bilateral involvement.11 However, we noted bilateral renal infection in two cases: EPN on one side and pyelonephritis on the other side, which aligns with Karthikeyan et al. who found it in one-tenth of their cases.13 It is not known why EPN predominantly involves the left kidney, or why it occurs on one side and pyelonephritis in the other in the same patient.
The mechanism of gas formation in EPN is widely attributed to glucose fermentation by enteric bacteria.3,14 The pathogenesis of EPN may also include infection by gas-forming organisms, tissue ischaemia, anaerobic environments and defective immune mechanisms.3 Vesicoureteric reflux also contributes.4 Obstruction of renoureteral units by stones, papillary necrosis or tumours can predispose to EPN and stones may act as a nidus for infection.1–3,5
Patients with EPN present with the typical features of an upper urinary tract infection (e.g. fever, renal-angle pain or tenderness, and vomiting).1–3,6,7 Occasionally they have altered consciousness and shock.3,14 Virtually no feature is diagnostic of EPN. The clinical presentation of fever, loin pain and vomiting in our series is comparable with other published series.11,13–16 Asymptomatic cases are occasionally identified during investigation for some other reasons.17
Neutrophilic leucocytosis, high ESR, high CRP and poor glycaemic status are consistent features as most EPN cases have DM.1–3,13–16 Occasionally an episode of EPN unmasks previously undiagnosed DM,3,18 which happened with one of our patients. E. coli is the most commonly isolated organism in most series.1–3,6,7,13–16 In our series, two patients had fungal growth (one case each of C. albicans and non-albicans Candida) on urine culture, but their blood remained sterile. Both were put on antibiotics after sending their urine and blood for cultures (as per hospital protocol); as a result of their clinical responses and urine culture reports, we did not add antifungals. We assumed that the fungal growth was facilitated by indwelling catheters. EPN caused by Candida has been treated with antifungals and nephrectomy.19 A fifth of our patients had no urine isolates, which is not unusual, especially in patients who receive antibiotics before seeking medical care,1 or who are transferred from other centres where they had been treated. However, ESBL-positive E. coli was cultured in pus collected during nephrectomy from one patient.
Bacteraemia, AKI and electrolyte imbalances were important complications in our series. In other studies, up to 75% of EPN cases were complicated by AKI.1,2 Our patients stayed in hospital for 7–31 days and most of them received antibiotics for 4 weeks, which was consistent with another report.1 None of our patients required transfer to the intensive care unit (ICU), which may relate to the predominantly less severe cases (class 2; 80%) in our series. Two patients with class 3B EPN (Figure 1) and one with class 2 EPN with gross renal parenchymal destruction (Figure 2) required nephrectomy. Huang and Tseng concluded that class 1, 2 and less aggressive presentations of class 3 and 4 EPN cases (with 1 out of 4 risk factors including shock, altered sensorium, AKI and thrombocytopaenia) may be managed by antibiotics and percutaneous drainage, while fulminant presentations of class 3 and 4 cases (2 or more risk factors) merit nephrectomy.3 Successful medical management of severe class 4 EPN is reported and many investigators nowadays use nephron-sparing strategies with favourable outcomes.20,21
Mortality associated with EPN has reduced largely in the last two or three decades. Poor prognostic features include altered conscious level and shock during admission, haematuria, proteinuria, hypoalbuminaemia, thrombocytopaenia, uraemia and the requirement for haemodialysis, polymicrobial infection, bacteraemia and extension of infection to perinephric areas.2,3 Our series had zero mortality, although some risk factors were present, such as low platelet count, hypoalbuminaemia, altered sensorium, uraemia and the requirement for haemodialysis. Irfaan et al. reported a series of 20 EPN patients, of whom nearly half had altered sensorium and a quarter had shock, without any mortality.14 Many factors contribute to the improved outcomes in EPN, including wide availability of CT scan facilities, early detection and less advanced radiological classes of EPN, aggressive resuscitative measures, rapid glycaemic control using intravenous insulin, early administration of effective, broad-spectrum intravenous antibiotics, interventional radiology, minimally invasive intervention techniques1 and a multidisciplinary team approach.2
Our study had some limitations: a small number of patients, retrospective evaluation, and location in a single centre. However, most other EPN series also have relatively small patient numbers and mostly evaluate hospital records retrospectively.1,2,5,7,11,14 A protocol-based, multicentre, prospective study with a larger sample may inform clinical practice and help establish management guidelines for EPN patients.
In conclusion, EPN occurred predominantly in females in this study, and all patients were diabetic. Fever, loin pain, vomiting and dysuria were common. Two-thirds of patients had AKI and a fifth required surgical intervention, including nephrectomy. There were no deaths. Although conservative management of EPN shows promising outcomes, we emphasise that interventions should not be delayed (as and when indicated) and may be life-saving. A high index of suspicion is warranted in appropriate common clinical scenarios, especially in patients with DM who respond slowly to antibiotic treatment for pyelonephritis, so that a CT scan can be performed early and an appropriate management strategy instituted to improve outcome. 
Acknowledgement
The authors would like to express their thanks to Samira Humaira Habib, Senior Research Officer, Health Economics Unit, Bangladesh Diabetic Somiti (BADAS), Dhaka, Bangladesh for analysing the data.
1 Boakes E, Batura D. Deriving a management algorithm for emphysematous pyelonephritis: can we rely on minimally invasive strategies or should we be opting for earlier nephrectomy? Int Urol Nephrol 2017; 49(12): 2127–36.
2 Sokhal AK, Kumar M, Purkait B et al. Emphysematous pyelonephritis: changing trend of clinical spectrum, pathogenesis, management and outcome. Turk J Urol 2017; 43(2): 202–9.
3 Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis and pathogenesis. Arch Intern Med 2000; 160: 797–805.
4 Pakkyara A, Jha A, Al Salmi I et al. Gas in the kidney in asymptomatic Escherichia coli urinary tract infections in a patient with severe vesicoureteral reflex. Saudi J Kidney Dis Transpl 2019; 30(3): 706–9.
5 Lu YC, Hong JH, Chiang BJ et al. Recommended initial antimicrobial therapy for emphysematous pyelonephritis: 51 cases and 14-year-experience of a tertiary referral center. Medicine (Baltimore) 2016; 95(21): e3573.
6 Wu VC, Fang CC, Li WY et al. Candida tropicalis-associated bilateral renal papillary necrosis and emphysematous pyelonephritis. Clin Nephrol 2004; 62(6): 473–5.
7 Jain A, Manikandan R, Dorairajan LN et al. Emphysematous pyelonephritis: Does a standard management algorithm and a prognostic scoring model optimize patient outcomes? Urol Ann 2019; 11(4): 414–20.
8 Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter (Suppl.) 2012; 2: 1–138.
9 Kelly HA, MacCallum WG. Pneumaturia. J Am Med Ass 1898; 31: 375–81.
10 Schultz EH Jr, Klorfein EH. Emphysematous pyelonephritis. J Urol 1962; 87: 762–6.
11 Misgar RA, Mubarik I, Wani AI et al. Emphysematous pyelonephritis: a 10-year experience with 26 cases. Indian J Endocrinol Metab 2016; 20(4): 475–80.
12 Wang Q, Sun M, Ma C et al. Emphysematous pyelonephritis and cystitis in a patient with uremia and anuria: A case report and literature review. Medicine 2018; 97: 45(e11272).
13 Karthikeyan VS, Manohar CMS, Mallya A et al. Clinical profile and successful outcomes of conservative and minimally invasive treatment of emphysematous pyelonephritis. Cent European J Urol 2018; 71: 228–33.
14 Irfaan AM, Shaikh NA, Jamshaid A et al. Emphysematous pyelonephritis: a single-center review. Pak J Med Sci 2020; 36(1) (Special Suppl. ICON 2020): S83–6.
15 Eswarappa M, Suryadevara S, John MM et al. Emphysematous pyelonephritis case series from South India. Kid Int Reports 2018; 3: 950–5.
16 Bhat RA, Khan I, Khan I et al. Emphysematous pyelonephritis: outcome with conservative management. Indian J Nephrol 2013; 23(6): 444–7.
17 Yeung AMH, Cheng CH, Chu PSK et al. A rare case of asymptomatic emphysematous pyelonephritis. Urol Case Rep 2019; 26: 100962.
18 Okunowo BO, Omidiji OA, Jeje EA et al. My flanks aches: emphysematous pyelonephritis in a newly diagnosed case of diabetes mellitus. Niger Postgrad Med J 2020; 27: 59–62.
19 Mohamed AH, Mohamud HA. Emphysematous pyelonephritis caused by Candida species: a case report and outcome of 1-year follow-up. Urol Case Rep 2020; 30: 101113.
20 Kalathia J, Chipde SS, Agrawal S et al. Nephron-sparing surgery in case of emphysematous pyelonephritis. Urol Ann 2015; 7: 504–6.
21 Dutta D, Shivaprasad KS, Kumar M et al. Conservative management of severe bilateral emphysematous pyelonephritis: case series and review of literature. Indian J Endocrinol Metab 2013; 17(Suppl.1): S329–32.
