Snake bites are an important yet neglected tropical disease. Snake bite envenoming (SBE) has a global burden of 2.7 million people every year with an annual mortality of 81,000–138,000 people, with nearly 400,000 surviving victims affected by permanent physical disabilities that reduce their quality of life.1 In spite of heavy morbidity and mortality, SBE has failed to attract the attention of clinicians globally. Exposure to more published data on this issue could hopefully lead to global participation in research, as well as developing healthcare infrastructure in the management of snake bites.
Pit viper of the genus Hypnale is called hump-nosed pit viper (HNV) or ‘Merrem’s hump-nosed pit viper’ (Hypnale hypnale) (Figure 1 and Supplementary Figure 1). This snake, which was once believed to be mildly venomous, is now considered as venomous as the ‘Big Four’ venomous snakes, namely the common krait (Bungarus caeruleus), Russell’s viper (Daboia russelii), Indian saw-scaled viper (Echis carinatus) and Indian cobra (Naja naja),which are found in India, Sri Lanka and other Asian countries. Out of the three species of the genus Hypnale into which this viper belongs, the H. hypnale is commonly seen in the Western Ghats of South India and Sri Lanka. The other two species, Hypnale nepa and Hypnale zara, are exclusively seen in Sri Lanka.2 H. hypnale is a small viper that is also known by several indigenous native names based on regional languages, namely kopi viriyan (coffee snake) in Tamil, churrutta in Malayalam3 and ‘chatte kandodi’ or ‘thoudu kandodi’ in Tulu, the local language of coastal Karnataka, India and Polon Thelissa/Kunakatuwa/Gata Polaga, in Sinhala. Though this venomous snake is endemic to a few geographical regions mentioned above, it is time to learn about this snake envenomation owing to its peculiar type of coagulopathy. Inclusion of this viper under the category of most significant venomous snakes by the World Health Organization (WHO) has helped raise awareness in clinicians across the globe. An extensive search on the prevalence of H. hypnale species contributing to the total burden of venomous snake bites did not yield any results except for a case series published by several researchers from Sri Lanka where burden of these snake bites is quite high.4–6
Figure 1 Hypnale hypnale, the snout is pointed and turned upwards ending in a hump. Image courtesy of Mr Mohit Shenoy K, Mangalore, India

The interesting aspect of the Hypnale envenomation is ascribed to the coagulopathy it produces, which is otherwise known as Hypnale coagulopathy. Coagulopathy is a disorder affecting the coagulation of blood resulting from the over or under activity of the coagulation pathway. The haemostatic dysfunction is highly variable and unpredictable in Hypnale coagulopathy. Coagulopathy could vary from a very mild and clinically insignificant manifestation in the form of slight laboratory derangement of clotting, to very serious life-threatening bleeding. Any test that indicates derangement of clotting confirms the possibility of coagulopathy. However, the sensitivity of the available standard diagnostic tests to identify Hypnale coagulopathy is equivocal and not very reliable.4–6
In this paper, an effort has been made to highlight Hypnale coagulopathy’s unique features compared with other Viperidae to enrich the available literature on Hypnale envenomation.
Incidence and prevalence of H. hypnale coagulopathy
In 1821, John Davy7 provided the first published report of serious envenomation by H. hypnale bite in the form of swelling and bleeding. Since then, there have been several publications on the envenomation caused by the HNV, mostly from Sri Lanka.4–6 The available studies show wide variation in the incidences of coagulopathy, varying from 4–39%.8 The clinical manifestations and diagnostic tests of coagulopathy due to H. hypnale envenomation are highly unpredictable, inconsistent and contradicting. This makes the study of this pit viper envenomation unique.
In a descriptive observational study involving 1,543 patients of HNV bite carried out at five major hospitals in Sri Lanka, 59 (3.8%) patients were found to have some sort of coagulopathy.4 A study involving 80 HNV bites found that whole blood clotting test in 20 minutes (WBCT20 or 20WBCT) only detected coagulopathy in one patient. The final conclusion in the study was that the coagulopathy seen in HNV envenomation is usually mild and would not be detected by WBCT20. In a WBCT20 test, 1–2 ml of venous blood is added to a clean, dry glass bottle or vial and is allowed to stand at room temperature for 20 minutes. The container is then inverted and the presence or absence of a complete clot is recorded. When no clot is formed and blood remains in the liquid state, the test is said to be positive, indicating the presence of coagulopathy. This study also showed that in such cases of mild envenomation, the coagulopathy could still be diagnosed with other laboratory tests, such as a mild elevation of international normalised ratio, low fibrinogen, and factor V and factor VIII levels.8
However, in a study that contradicts the findings of the above, Premawardena et al.6 reported that H. hypnale envenomation leads to a severe form of coagulopathy with continued oozing of blood from the bite site. They found 12 (21.4%) out of 56 patients with H. hypnale bites had prolonged clotting time, increased fibrinogen degradation products and low fibrinogen levels. Excessive fibrinolysis was found to be the main cause of coagulopathy. In contrast to this observation, Sellahewa et al.9 in their prospective clinical study involving 62 patients bitten by H. hypnale reported no evidence of systemic envenomation including coagulopathy. All their patients had only local clinical features. However, the findings of an observational study carried out in Kerala, South India on snake bite envenomation was totally different. It found that 25% of patients who had coagulopathy were bitten by HNV. The same study also showed 96.4% of all the patients with HNV envenomation had some features of coagulopathy.10
Pathophysiology of HNV coagulopathy
Venom-induced consumption coagulopathy (VICC) is the most common and salient feature of systemic envenomation due to viper bites. VICC occurs as a result of the toxins present in the snake venom that are pro-coagulant in nature and activate the clotting pathway, eventually leading to the depletion of the clotting factors by virtue of consumption and increasing the risk of bleeding.8 These toxins differ based on the snake species and they activate different parts of the clotting pathway by targeting different clotting factors. The clinical severity depends on the clotting factors that are targeted. Therefore, the mechanism of VICC differs based on the species of the snake; the toxins could act as prothrombin activators, factor V and factor X activators and thrombin-like enzymes (TLEs). The venom of H. hypnale seems to contain TLEs that are otherwise known as fibrinogenases.11 The precise nature of the coagulopathy in HNV bites is not fully understood. However, it is believed to be due to the VICC known as ‘Hypnale coagulopathy’.9 There are several types of snake venom TLEs (SV-TLEs) in HNV venom and most of them are zinc metalloproteinases, which are unique to the venom of H. hypnale.12–14 The SV-TLEs cleave different chains of fibrinogen molecule into various fibrinopeptides without producing fibrin monomer (Figure 2). Therefore, in Hypnale coagulopathy there are low levels of fibrinogen due to fibrinogenolysis without the production of fibrin. Thus, excessive fibrinogenolysis could be the key factor in the pathogenesis of Hypnale coagulopathy indicated by the prolonged clotting time, increased levels of fibrinogen degradation products and decreased levels of fibrinogen.6,8,15 Low levels of factor V and factor VIII are the other characteristic features of Hypnale coagulopathy. Hypnale coagulopathy is usually a mild type of coagulopathy and is not detected by WBCT20.7 However, there are reports of H. hypnale envenomation with undetectable fibrinogen along with a deficiency of other clotting factors leading to serious bleeding complications as seen in other Viperidae envenomation.6,8,11 The activation of factor VIII and eventually its depletion found in these cases of severe envenomation could be due to the SV-TLEs in the Hypnale venom.
Figure 2 A schematic representation of the pro-coagulant toxin effect of Hypnale venom. PL: platelet phosphor lipids; SV-TLE: snake venom thrombin-like enzyme
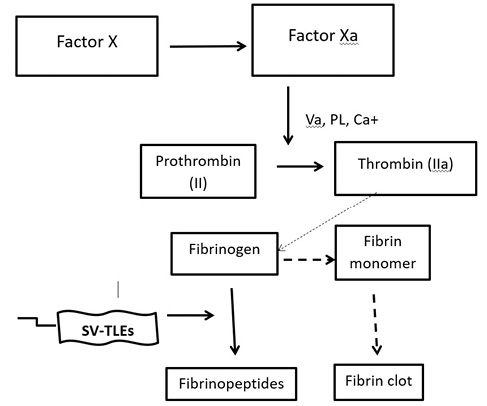
Near normal levels of factor V clotting factor seems to be another characteristic feature of Hypnale coagulopathy compared with other VICCs. The possibility of occurrence of several types of SV-TLEs in the Hypnale venom could be the explanation for the selective activation of the different clotting factors and their varied depletion.8,16
Several patients with Hypnale coagulopathy did not complain of any local symptoms, and this could be due to the fact that SV-TLEs in the Hypnale venom are mainly acidic with multiple isoforms. In other Viperidae it is the basic fraction, namely hyaluronidase and L-amino acid oxidase, that leads to local necrosis.6,12,13 It has also been demonstrated that HNV venom has a weaker phospholipase A2 activity, an enzyme responsible for the local inflammatory features, compared with other Viperidae.17
Hypnale coagulopathy could also lead to acute kidney injury (AKI), though the primary nephrotoxicity by the Hypnale venom could be another major causative factor in these cases of AKI.18,19
Hypnale venom
The Hypnale venom when subjected to fractionation by ion exchange high performance liquid chromatography reveals several protein fractions with enzyme activities, namely proteases, phospholipase A2, alkaline phosphomonoesterase, phosphodiesterase and 5’ nucleotidase. The unique SV-TLE is present in multiple isoforms. In comparison with other Viperidae, this venom shows 5’ nucleotidase and phosphomonoesterase existing in both basic and acidic isoforms.13 The venom analysis further reveals that there are two subtypes of phospholipase A2 (E6-PLA2 and W6-PLA2) that constitute the major fraction of venom composition (40.1%). The other constituents of this snake venom are metalloproteases (36.9%), l-amino acid oxidase (11.9%), C-type lectins (5.5%), serine proteases (3.3%) and others (2.3%).19 Thus, HNV venom is considered to have pro-coagulant, nephrotoxic, myotoxic and cytotoxic effects, and the fibrinogen degradation products result in bleeding tendency due to their anti-haemostatic effects. Studies have also revealed that the neurotoxic effect is quite weak in this venom.3,19 The haematological manifestations are mainly due to the presence of TLE, proteases, phospholipase A2, L-amino acid oxidase and hyaluronidase in HNV venom. It is interesting to know, therapeutically the Indian polyvalent antivenom has no effect on the pro-coagulant and phospholipase effect of the HNV venom.3,4,20,21
Clinical manifestations of H. hypnale coagulopathy
Hypnale coagulopathy is generally asymptomatic as shown in the study carried out by Wijewantha et al.4 at five major hospitals in Sri Lanka. Hypnale coagulopathy is also not a common feature of H. hypnale envenomation; however, it is the second most common feature after the local clinical features.3,8 Hypnale coagulopathy could be minor bleeding from the venepuncture sites, oozing of blood from the site of the bite or serious spontaneous systemic haemorrhage, such as gum bleeding, haematuria, haematemesis, overt gastrointestinal bleeding and retroperitoneal haemorrhage.
Serious systemic manifestations of Hypnale coagulopathy
There are several published reports of serious systemic manifestations of Hypnale coagulopathy, which are given below.
Microangiopathic haemolytic anaemia
Namal Rathnayaka et al.22 described Hypnale coagulopathy presenting as prolonged coagulopathy along with ecchymosis and microangiopathic haemolytic anaemia following H. hypnale bite. It is interesting to note that this is not a common feature seen in other viper envenomation.
Delayed-onset coagulopathy
Haematuria
There are reports of patients developing haematuria as late as 48 hours following the bite with normal WBCT20 in the initial 24 hours. In these cases, the clotting tests were found to be abnormal only after the onset of haematuria.6,19
Retroperitoneal haemorrhage
Retroperitoneal haemorrhage following a HNV bite has been reported presenting as late as 5 weeks after the bite.23
Thrombotic microangiopathy without classical VICC
A rare but interesting presentation of HNV bite as thrombotic microangiopathy without the classical VICC has also been reported. The patient had thrombocytopenic purpura-like syndrome, characterised by thrombocytopenia, renal impairment, neurological dysfunction, fever and microangiopathic haemolysis.24
Fatal pulmonary haemorrhage
There is a recent publication on a fatal case of Hypnale coagulopathy in which the patient succumbed to pulmonary haemorrhage along with other systemic bleeding manifestations, including thrombotic microangiopathy.25
Disseminated intravascular coagulation
Kumar et al.10 in their observational study on venomous snake bites carried out in Kerala, South India, found that 17 out of 89 patients who had features of disseminated intravascular coagulation were bitten by HNV.
Asymptomatic persistent coagulopathy
In many patients asymptomatic coagulopathy is seen in the form of non-coagulable blood for several days. The coagulopathy gets resolved gradually over a period of a few days spontaneously without any intervention. Reassuring the patients and their relatives during this period is most important. Similar observations have been reported by Dorji26 in their snake bite patients.
It is known that the clinical manifestations of snake envenomation can depend on several factors, such as the season and fasting or fed state of the snake, as well as the quantity of the snake venom injected. Other factors include the site of bite, venom injection directly into the veins, whether the bite site is covered by cloth and the type of cloth, completeness of bite, the contact time during bite, time taken to reach a healthcare set up and, finally, actual administration of antivenom. Other variables include the age of the snake, size of the snake along with the weight of the victim and immobilisation of the victim following the bite. The H. hypnale bite could be no exception to this.
Management of Hypnale coagulopathy
It is imperative to confirm the HNV bite before contemplating the treatment, as the treatment plan differs to other viper envenomation.
Confirmation of HNV envenomation and detection of coagulopathy
Visual examination of the snake brought to the hospital either dead or alive should be carried out. However, clinicians should discourage the age-old habit of relatives bringing the killed snakes for identification, instead relying more on the photographs, video or internet-based images of the species of the snakes in a bid to protect the all-important snake population. Encouraging the relatives to release the live snakes to the wild after due identification is another way of snake conservation.8,19
In an endemic area, an asymptomatic patient developing laboratory evidence of coagulopathy late in the course of treatment should raise the suspicion of Hypnale envenomation, though this feature could be seen in other viper envenomation due to slow venom absorption.8,19
There is no single confirmatory and specific laboratory test that will detect Hypnale coagulopathy as the sensitivity of almost all the available standard diagnostic tests to identify this coagulopathy are equivocal and unreliable.4–6 Therefore, the treating clinicians are compelled to rely on the following multiple laboratory abnormalities to diagnose coagulopathy, although many of them are seen in VICC due to other vipers.
- Prolonged clotting time, increased levels of fibrinogen degradation products and decreased levels of fibrinogen.6,8,15
- Mild elevation of international normalised ratio, low but detectable levels of fibrinogen, and low levels of factor V and factor VIII are the other characteristic features of this Hypnale coagulopathy.
- WBCT20 is a good test to diagnose VICC, however, in mild cases Hypnale coagulopathy it is often negative.8
- Detection in the blood of specific venom antigens using immunologically-based techniques, such as enzyme immunoassay,8 though these are not available widely at present.
Non-efficacious polyvalent antivenom
The polyvalent antivenom available in India, Sri Lanka and other South Asian countries has been found to be ineffective against Hypnale envenomation and this is the main drawback in the management of Hypnale coagulopathy. The antivenom could be ineffective because the venom of HNV is not incorporated in the process of preparation of this antivenom.4 Sellahewa et al.19 reiterated this fact in their study in Sri Lanka. Furthermore, in the state of Kerala, South India, a study on H. hypnale snake envenomation showed that four out of five (80%) cases had persistent coagulopathy for more than 18 hours despite administration of polyvalent antivenom, clearly indicating its ineffectiveness in reversing the Hypnale coagulopathy.27 These clinical observations have also been confirmed in in vitro studies, which demonstrated that Hypnale venom enzyme activity was not being neutralised by the locally available polyvalent antivenoms.3
Management options available at present
The current management options for Hypnale envenomation are:
- In the majority of cases, the local symptoms could be managed with simple analgesics, such as paracetamol.19
- Withhold the use of polyvalent antivenom in proven cases of HNV bite in view of its ineffectiveness and risk of allergic reactions.27
- However, when the patient has overt bleeding tendency and there is laboratory evidence of severe coagulopathy withholding antivenom therapy would not be a wise clinical decision as there are no confirmation tests available to diagnose H. hypnale envenomation with absolute certainty.
- Observe the patient in the hospital for at least 48 hours following HNV bite to look for the development of delayed onset of coagulopathy.4,19
- Use isotonic saline with or without fresh frozen plasma in order to prevent AKI.19
- Use fresh frozen plasma at a dose of 15 ml/kg body weight every 4 hours until the reversal of coagulopathy.19 However, there is always a clinical dilemma about the duration of this treatment when the patient is asymptomatic but for the non-coagulable blood. A recent report from Sri Lanka has shown that fresh frozen plasma is not only ineffective in H. hypnale coagulopathy but also could worsen it.28
- Many studies are underway to develop antivenom against Hypnale envenomation and a study by Tan et al.29 showed cross-neutralization of H. hypnale venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in an in vivo rodent model of envenomation, which is promising.
A clinician’s dilemma
It is a dilemma whether a clinician should treat or not an asymptomatic patient who has been bitten by HNV with just laboratory evidence of coagulopathy. It could also be difficult for a clinician to convince an asymptomatic patient to stay in the hospital for observation and treatment. Reassurance of the patient and their relatives with judicious observation for any clinical signs of bleeding tendency is the need of the hour.
A summary of the salient features of Hypnale coagulopathy compared with coagulopathy from other Viperidae has been presented in Table 1, and Table 2 shows the compilation of serious systemic manifestions of Hypnale coagulopathy.
There is also a need for the development of a cost-effective rapid diagnostic test to diagnose Hypnale envenomation. In the absence of specific antivenom, a suitable and universally acceptable snake bite management protocol for this viper envenomation should be made available. The use of Indian polyvalent antivenom, which has shown poor efficacy in this type of snake envenomation, should be discouraged, keeping in mind the ineffectiveness, cost and risk of allergic reactions. 
Supplementary Figure 1 and Acknowledgements are available with the online version of this paper, which can be accessed at https://www.rcpe.ac.uk/journal
References
1 Williams DJ, Faiz MA, Abela-Ridder B et al. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Negl Trop Dis 2019; 13: e0007059.
2 Maduwage K, Silva A, Manamendra-Arachchi K et al. A taxonomic revision of South Asian hump nosed pit vipers (Squamata:Viperidae:Hypnale). Zootaxia 2009; 2232: 1–28.
3 Shivanthan MC, Yudhishdran J, Navinan R et al. Hump-nosed viper bite: an important but under-recognized cause of systemic envenoming. J Venom Anim Toxins Incl Trop Dis 2014; 20: 24.
4 Wijewantha HS, Sellahewa KH. Hump nosed viper bite in Sri Lanka-descriptive observational study of 1543 cases. Asian Pac J Trop Med 2010; 3: 902–5.
5 Maduwage K, Isbister GK, Silva A et al. Epidemiology and clinical effects of hump-nosed pit viper (Genus: Hypnale) envenoming in Sri Lanka. Toxicon 2013; 61: 11–5.
6 Premawardena AP, Seneviratne SL, Gunatilake SB et al. Excessive fibrinolysis: the coagulopathy following Merrem’s hump-nosed viper (Hypnale hypnale) bites. Am J Trop Med Hyg 1998; 58: 821–3.
7 Davy J. An Account of the Interior of Ceylon, and of its Inhabitants. London: Longman, Hurst, Rees and Brown; 1821.
8 Maduwage K, Scorgie FE, Silva A et al. Hump-nosed pit viper (Hypnale hypnale) envenoming causes mild coagulopathy with incomplete clotting factor consumption. Clin Toxicol (Phila) 2013; 51: 527–31.
9 Sellahewa KH, Kumararatne MP. Envenomation by the hump-nosed viper (Hypnale hypnale). Am J Trop Med Hyg 1994; 51: 823–5.
10 Kumar KS, Narayanan S, Udayabhaskaran V et al. Clinical and epidemiologic profile and predictors of outcome of poisonous snake bites – an analysis of 1,500 cases from a tertiary care center in Malabar, North Kerala, India. Int J Gen Med 2018; 11: 209–16.
11 Berling I, Isbister GK. Hematologic effects and complications of snake envenoming. Transfus Med Rev. 2015; 29: 82–9.
12 Tan NH, Ponnudurai G. Biochemical characterization of snake venoms. In: Gopalakrishnakone P, Tan CK, editors. Recent Advances in Toxinology Research. Singapore: Venom & Toxin Research Group; 1992. pp. 210–59.
13 Tan CH, Sim SM, Gnanathasan CA et al. Enzymatic and toxinological activities of Hypnale hypnale (hump-nosed pit viper) venom and its fractionation by ion exchange high performance liquid chromatography. J Venom Anim Toxins 2011; 17: 473–85.
14 Isbister GK, Woods D, Alley S et al. Endogenous thrombin potential as a novel method for the characterization of pro-coagulant snake venoms and the efficacy of antivenom. Toxicon 2010; 56: 75–85.
15 Isbister GK. Snakebite doesn’t cause disseminated intravascular coagulation: coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost 2010; 36: 444–51.
16 Isbister GK, Scorgie FE, O’Leary MA et al. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J Thromb Haemost 2010; 8: 2504–13.
17 Maduwage K, Hodgson WC, Konstantakopoulos N et al. The in vitro toxicity of venoms from South Asian hump-nosed pit vipers (Viperidae: Hypnale). J Venom Res 2011; 2: 17–23.
18 Herath N, Wazil A, Kularatne S et al. Thrombotic microangiopathy and acute kidney injury in hump-nosed viper (Hypnale species) envenoming: a descriptive study in Sri Lanka. Toxicon 2012; 60: 61–5.
19 Sellahewa KH. Hump-nosed pit viper bite in Sri Lanka–unravelling an enigma. J Trop Dis 2013; 1: 114.
20 Tan CH, Tan NH, Sim SM et al. Proteomic investigation of Sri Lankan hump-nosed pit viper (Hypnale hypnale) venom. Toxicon 2015; 93: 164–70.
21 Simpson ID, Norris RL. Snakes of medical importance in India: is the concept of the “Big 4” still relevant and useful? Wilderness Environ Med 2007; 18: 2–9.
22 Namal Rathnayaka RMMK, Kularatne SAM, Ranathunga AN et al. Prolonged coagulopathy, ecchymoses, and microangiopathic hemolytic anemia following hump-nosed pit viper (Hypnale hypnale) bite in Sri Lanka. Wilderness Environ Med 2017; 28: 253–8.
23 Sunanda H, Tilakaratne S, Coomaraswamy W. Retroperitoneal haemorrhage following a hump-nosed viper (Hypnale hypnale) bite: a late presentation. Galle Med J 2010; 15: 41–2.
24 Withana M, Rodrigo C, Gnanathasan A et al. Presumptive thrombotic thrombocytopenic purpura following a hump-nosed viper (Hypnale hypnale) bite: a case report. J Venom Anim Toxins Incl Trop Dis 2014; 20: 26.
25 Rathnayaka RMMKN, Ranathunga PEAN, Kularatne SAM. Systemic bleeding including pulmonary haemorrhage following hump-nosed pit viper (Hypnale hypnale) envenoming: a case report from Sri Lanka. Toxicon 2019; pii: S0041-0101(19)30451-9.
26 Dorji T. Is anti-snake venom required for all snakebites: a case report. Clin Case Rep 2019; 8: 194–7.
27 Joseph JK, Simpson ID, Menon NC et al. First authenticated cases of life-threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans R Soc Trop Med Hyg 2007; 101: 85–90.
28 Kumara H, Seneviratne N, Jayaratne DS et al. Severe coagulopathy in Merrem’s hump-nosed pit viper (Hypnale hypnale) envenoming unresponsive to fresh frozen plasma: a case report. Toxicon 2019; 163: 19–22.
29 Tan CH, Leong PK, Fung SY et al. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop 2011; 117: 119–24.