Late-onset hypogonadism (LOH) remains a debatable biochemical syndrome associated with reduced androgen levels in ageing men.1 Known by various names, such as androgen deficiency in ageing male (ADAM) and andropause, LOH is characterised by symptoms and signs related to testosterone deficiency. As these symptoms are mostly non-specific, biochemical confirmation of the diagnosis by measurement of early morning serum testosterone levels remains a prerequisite for further management.1
Patient-centred care is accepted in the management of chronic endocrine conditions such as diabetes mellitus2 and one can argue a similar approach needs to be adapted for men who are clinically and biochemically deemed to have LOH. Men experiencing signs and symptoms associated with age-related decline in testosterone levels potentially endure unique biomedical and psychosocial challenges. The expectations from therapeutic intervention and the risk-benefit profile from the treatment might differ, further reinforcing the importance of individualised decision making.3 This article discusses the complex issues surrounding the management of LOH in light of patient-centric thresholds and therapeutic targets.
Threshold for evaluation
The primary, and ‘rate limiting’ step for diagnosis of LOH remains a clinical suspicion, rather than adapting a practically non-viable approach of universal biochemical screening. Clinical evaluation, therefore, should be comprehensive enough not to miss any person living with androgen deficiency.4 While good screening questionnaires such as Androgen Deficiency in Ageing Male (ADAM) are available to quantify the severity of hypogonadism, it stills needs awareness as well as a high index of clinical suspicion to screen patients displaying signs and symptoms associated with low testosterone levels.5,6
The symptoms and signs of LOH can be classified as physical, psycho-cognitive, and social (Box 1).1,7 This framework makes it easy for the practicing clinician to take a comprehensive history in a systematic manner and conduct a focused physical examination. The patient may present with ‘depressive symptoms’ which do not meet the criteria for major depressive disorders. A few thoughtful questions may help elicit a diagnosis of testosterone deficiency.4,7 Recent changes in liking for music (for example from pop music to ghazals), clothes (less focus on grooming, bright colours) and driving (slower speed of driving) may be subtle pointers towards an andropause state.
Box 1 Clinical features of late-onset hypogonadism
Physical
- Dyspnea on exertion
- Low energy levels
- Increased sweating
- Loss of body hair
- Sarcopenia
- Osteopenia/osteoporosis
- Loss of height
Psychocognitive
- Hot flashes
- Loss of concentration
- Change in mood
Sexual
- Reduced sexual thoughts
- Progressive decline of libido
- Reduced or absent morning erections
- Erectile dysfunction
It is of utmost importance to ensure that all patients displaying features associated with low testosterone levels undergo further clinical evaluation to exclude possible reversible aetiologies contributing to a hypogonadal state. LOH is a diagnosis of exclusion and a few of the important differential diagnoses which need to be considered are listed in Box 2.8,9 The 4x4 rubric presented in this table helps the busy clinician (and medical students) understand the wide spectrum of testosterone physiology and action.
Box 2 Differential diagnoses of late-onset hypogonadism
Endocrine
- Hypothyroidism
- Hyperprolactinaemic
- Growth hormone deficiency
- Adrenal insufficiency
- Obstructive sleep apnoea
- Anaemia
- Vitamin D deficiency
- Obesity
- Depression
- Anxiety neurosis
- Bipolar disorder
- Adjustment disorder
- Heart failure
- HIV
- Chronic kidney disease
- Cirrhosis
- Neoplasm
- Medications such as beta blocker, anti-depressants, opioid analgesics, anabolic steroids
The next step in diagnosis of possible LOH remains a biochemical confirmation of low serum testosterone levels. One must be aware, however, of the various caveats and cautions related to testosterone measurement (Box 3).10Thoughtful counselling of the patient, and if required, the laboratory technician, helps minimise the potential pre-analytical errors in estimation. It must be reiterated here that the trigger for testing is a symptom or sign. Screening is not recommended in asymptomatic men.11,12
Box 3 Testosterone estimation: caveats and caution
- Take a drug history, particularly of antiandrogen and androgenic steroid use.
- Take a morning fasting sample. Be aware of diurnal variation, especially in younger men and impact of meals on testosterone.
- Ensure proper collection, storage and transport of sample.
- Be aware of conditions altering sex hormone binding globulin (SHBG) – to consider free testosterone.
- Use a validated assay with low coefficient of variation
- If severe hypogonadism is suspected, prefer an assay with a lower normal range.
- Advise free testosterone when indicated e.g. in liver disease and nephritic syndrome.
- Ensure proper interpretation of results.
- Correlate results with clinical picture; do not view them in isolation.
- Calculate free testosterone index if needed.
Threshold for treatment
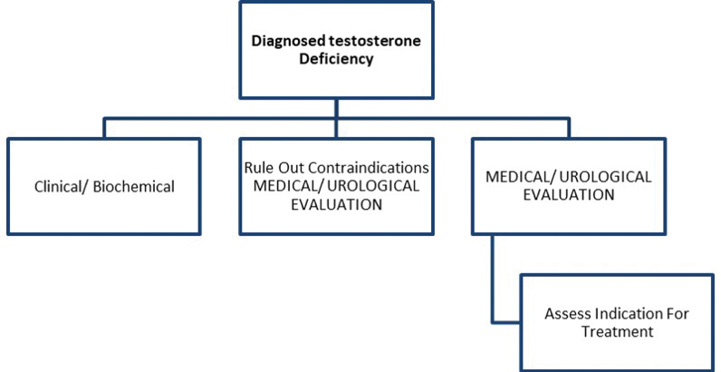
The cornerstone of management of LOH remains lifestyle modification to mitigate the impact of non-gonadal and metabolic dysregulated states such as obesity, hypertension and type 2 diabetes mellitus (DM), apart from judicious use of testosterone replacement if deemed appropriate.13One must be aware, however, of the indication of treatment (symptomatic hypogonadism, biochemical hypogonadism, or both), the planned duration of therapy, expected adverse events, and monitoring schedule. All contraindications must be ruled out prior to starting hormone replacement therapy. A clear risk-benefit analysis, shared with the patient, is essential for the creation of a treatment plan. The patient’s wishes, needs, preferences and limitations must be considered during the process of informed decision making.14
The threshold for initiation of testosterone may vary and needs a patient-centric individualised approach.13 An individual with severe symptoms suggestive of testosterone deficiency, in whom other possible aetiologies (listed in Box 2) have been ruled out, and who faces significant personal/professional distress/disability due to his complaints, may be treated even if serum testosterone is in the lower range of normal.15 A therapeutic trial may be agreed upon, keeping relief of symptoms as a pre-decided endpoint. It must be noted that psychological and sexual symptoms respond faster to testosterone than erythropoiesis, metabolic and musculoskeletal health.16
In resource-constrained settings, such a therapeutic trial may be offered for a few months without detailed investigations, provided the treating endocrinologist is confident of the diagnosis of andropause, has ruled out other aetiologies of symptoms, and has confirmed that there are no contraindications.17 Various international guidelines use differing cut-offs to label testosterone deficiency. There is a need to take this discussion further and propose person-centred thresholds for intervention, based upon the spectrum, severity, and significance of symptoms, as well as the swiftness expected in their resolution.18 This heuristic is highlighted in Box 4.
Box 4 Individualised patient-centric thresholds for testosterone initiation. The ‘4S’ factors for symptoms
- A wide range of symptoms
- Musculoskeletal impairment
- Sudden onset
- Severe symptoms
- Causing significant personal/professional/social/medical distress
- Need for rapid resolution
- Expected benefit with therapy
Targets
The trigger for measurement of testosterone levels and possible therapeutic intervention remains clinical evaluation based on the signs and symptoms experienced by the patient. Use of ADAM questionnaires to provide an objective quantification of symptoms experienced can provide a baseline benchmark to evaluate response to therapy. The dose of testosterone replacement therapy (TRT) should be titrated to aim for serum testosterone in mid-normal range, apart from focusing on relief of hypogonadism-related symptoms.19 Obviously, the definition of well-being, and the balance between physical, psycho-cognitive, and sexual needs, remains subjective, with wide variations in response to be expected.
International guidelines suggest that the goal of testosterone replacement is to optimise cardiovascular and musculoskeletal health.20,21 While this is laudable, as the improvement in the cardiovascular and bone-health surrogate markers might be gradual and not overtly appreciable by the patients, alleviation in physical and sexual health-related symptoms remains crucial and ensures better compliance with therapy. It is also essential to keep a watch on blood pressure, haematocrit and prostate size while monitoring elderly men on testosterone therapy.
Techniques
An individualised and patient-centric approach remains the key for optimum management of individuals with andropause. As discussed in previous sections, hypogonadism can induce a wide array of symptoms varying from hot flashes, increased sweating, loss of libido, low energy levels and erectile dysfunction. Individuals differ on subjective response to symptoms experienced and the associated impact of hypogonadism on their quality of life. One patient might deem improvement in bone or muscle mass more important, while another might be more concerned with relief of distressing hot flashes or loss of libido. Individualised decision making with a focus on pre-agreed, symptoms-driven, end points can potentially help to achieve desired compliance with therapy and improve the eventual outcomes.22
It must also be emphasised that andropause management is not limited to testosterone replacement alone. Many complaints attributed to testosterone deficiency, such as fatiguability, sarcopenia, loss of bone mass, central obesity, and sexual dysfunction, might be due to other factors.23The LEMON mnemonic, used as a rubric for the evaluation of fatigue (lifestyle, endocrine, medical/metabolic, observer-induced or iatrogenic, and nutritional) reminds us of the need to promote a healthy lifestyle and optimise overall health.
Focus especially on the cardiovascular, metabolic, and endocrine health helps to improve quality of life, and may obviate, or reduce the need for testosterone replacement, as well as improve its efficiency.
Tools
Once the need for testosterone replacement has been agreed upon, the next step is to decide the testosterone preparation, or tools of therapy. Testosterone therapy is available in a variety of formulations, including oral capsules, transdermal gels, and injections of varying potency and duration.24 Table 1 lists the commonly available preparations in the market, their properties, advantages, and limitations. From personal experience, we propose the following heuristic for testosterone replacement in andropause (Figure 1). In current times, where there is a need to minimise contact of patients with the healthcare system, gels and capsules may be preferred.24
Table 1 Preparations for testosterone replacement therapy
|
|
|
|
|
|
|
|
Testosterone aqueous
|
25-50mg every 1-2 weeks
|
Less painful
|
Frequent injections
|
|
Testosterone enanthate
|
250mg every 2-3 weeks
|
Cost effective
|
- Wide fluctuations in T levels
- Fluctuation in mood/libido
- Multiple injections
- Relative higher risk of polycythaemias
|
|
Testosterone cypionate
|
200mg every 2-3 weeks
|
Cost effective
|
|
Testosterone propionate
|
100mg every 2 weeks
|
Cost effective
|
|
|
|
Testosterone undecanoate
|
1000mg every 10-14 weeks
|
Longer effects
Convenience
|
- Pain at the injection site
- Risk of venous thromboembolism
|
|
|
|
Testosterone undecanoate*
|
40-80mg BID/TID
with meals*
|
Ease of oral administration
|
|
|
|
|
Buccal, bio-adhesive,
testosterone tablets
|
30mg controlled release,
Bio-adhesive tablets BID
|
Mimics diurnal variation
Quick reversal
|
- Gum-related adverse events in 16% of treated men
|
|
|
|
Testosterone gel (Available in sachets, tubes, and pumps)
|
40-50mg on shoulders, upper arms, abdomen once daily in the morning
|
Easy application
|
|
|
Transdermal testosterone
patch
|
1 or 2 patches,
designed to nominally
deliver 5–10mg T over
24 hours applied every day on non-pressure areas
|
Mimics circadian rhythm, easy administration
|
- Skin irritation at the application site
- Cannot use for more than seven days
|
|
|
|
Surgical implants
|
2–6 (150-450mg) pellets implanted SC; every 3-6 months.
Dose and regimen vary with formulation
|
Dosing frequency Improved compliance
|
- Requires surgical incision for insertions
- Pellets may extrude spontaneously
|
|
*Oral testosterone undecanoate is 17-β undecylate molecule which is not hepatotoxic and the variability in absorption with fatty meals is negligible.
|
Figure 1 Algorithmic approach to testosterone replacement therapy in andropause
1a: Diagnosis
1b: Treatment
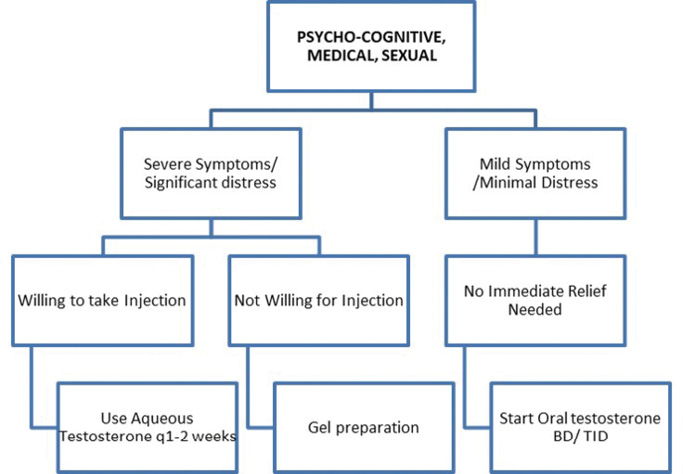
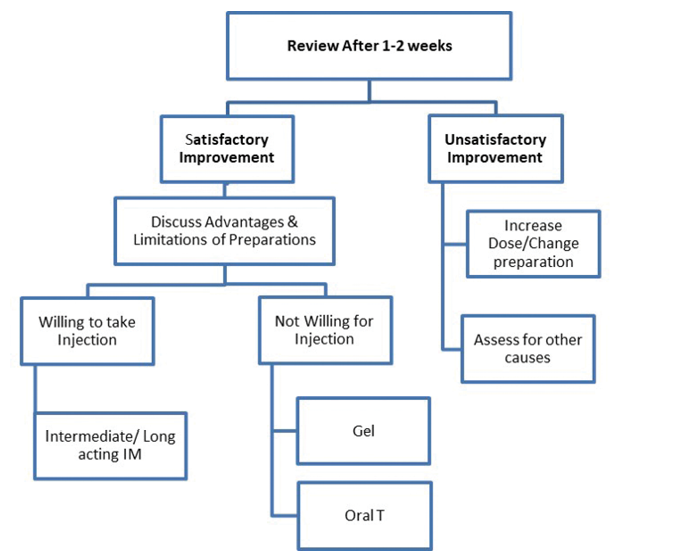
The duration of treatment may vary from person to person. Testosterone replacement for relief of hot flashes may be limited to months but should be continued for years if being prescribed to manage osteoporosis.12-14 As per current evidence, there is no level-one evidence derived from randomised controlled trials regarding the beneficial impact of TRT on prevention of atherosclerotic cardiovascular disease (ASCVD), although observational studies and systematic review support use of TRT in hypogonadal men with type 2 DM and/or metabolic syndrome. In persons who respond to testosterone, the drug should be continued for the long term. The role of monitoring for prostatic health, haematocrit and cardiovascular well-being cannot be over emphasised. Monitoring must be done as per the standard guidelines, to ensure patient safety.9,15,16 Last, though not least, physicians need to stay alert to subtle symptoms and signs of over-replacement of testosterone therapy (Box 5).
Box 5 Monitoring of testosterone replacement therapy
- Haematocrit
- Liver function tests
- Prostate specific antigen (PSA)
- Ultrasound prostate, if indicated
- Unexplained aggression/violence
- Sexual hyperactivity
- Male pattern alopecia, sudden onset or worsening
- Darkening of skin
- Gynaecomastia, sudden onset
Evidence from the European Male Ageing Study and the Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China) data suggests that low testosterone in elderly men is often secondary to nongonadal comorbidities that lead to suppression of luteinising hormone (LH) thereby reducing testosterone. However, this does lead to significant comorbidities such as exacerbating illness-associated anaemia, myopathy and frailty that may affect the quality of life in such individuals.17,18
Summary
This article describes a person-centric approach to the management of LOH. It specifically highlights the need to consider symptoms as the starting point, the lighthouse or milestones, and the target of treatment, while ensuring safety at all times.
References
1 Morales A, Lunenfeld B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. International Society for the Study of the Aging Male. The Aging Male 2002; 5: 74-86.
2 Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55: 1577-96. Published correction appears in Diabetologia 2013; 56: 680.
3 Brawer MK. Testosterone replacement in men with andropause: an overview. Rev Urol 2004; 6: S9-S15.
4 McBride JA, Carson CC, Coward RM. Diagnosis and management of testosterone deficiency. Asian J Androl 2015; 17: 177-86.
5 Mohamed O, Freundlich RE, Dakik HK et al. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res 2010; 22: 20-24.
6 Morley JE, Charlton E, Patrick P et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49: 1239-42.
7 Jakiel G, Makara-Studzinska M, Ciebiera M et al. Andropause – state of the art 2015 and review of selected aspects. Prz Menopauzalny 2015;14:1-6.
8 Hayes F, Dwyer A, Pitteloud N. Hypogonadotropic Hypogonadism (HH) and Gonadotropin Therapy. [Updated 2013 Nov 25]. In: Feingold KR, Anawalt B, Boyce A et al. (editors). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000.
9 Carnegie C. Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol 2004; 6: S3-S8.
10 Trost LW, Mulhall JP. Challenges in testosterone measurement, data interpretation, and methodological appraisal of interventional trials. J Sex Med 2016; 13: 1029-46.
11 Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting. Int J Clin Pract 2010; 64: 682-96.
12 Lawrence KL, Stewart F, Larson BM. Approaches to male hypogonadism in primary care. Nurse Pract 2017; 42: 32-37.
13 Maganty A, Shoag JE, Ramasamy R. Testosterone threshold – does one size fit all? Aging Male 2015; 18: 1-4.
14 Fode M, Salonia A, Minhas S et al. Late-onset hypogonadism and testosterone therapy – a summary of guidelines from the American Urological Association and the European Association of Urology. Eur Urol Focus 2019; 5: 539-44.
15 Behre HM, Kliesch S, Leifke E et al. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. Journal Clin Endocrinol Metab 1997; 82: 2386-90.
16 Tirabassi G, Biagioli A, Balercia G. Bone benefits of testosterone replacement therapy in male hypogonadism. Panminerva Med 2014; 56: 151-63.
17 Lee DM, O’Neill TW, Pye SR et al. The European Male Ageing Study (EMAS): design, methods and recruitment. Int J Androl 2009; 32: 11-24.
18 Cheng J, Han B, Li Q et al. Testosterone: relationships with metabolic disorders in men – an observational study from SPECT-China. Int J Endocrin 2017; 2017: 4547658.