Systemic lupus erythematosus (SLE) is an uncommon autoimmune disorder. Though the exact cause of this disease is unknown, rarely, it could be secondary to drugs. Common drugs implicated in causing drug-induced lupus (DIL) are isoniazid,1,2 hydralazine,1,3 procainamide1,4 and antiepileptics, such as phenytoin5 and carbamazepine.6 Levetiracetam is a relatively new antiepileptic, commonly used due to its fewer adverse effects. To date, we have not found any report of levetiracetam causing DIL. Here we present a case of a 62-year-old female who developed SLE after taking levetiracetam. As with this patient, DIL should be suspected if SLE presents at an atypical age, manifests as typical rash and resolves on withdrawal of the drug.
Case presentation
This is a case of a 62-year-old female, hypertensive and hypothyroid for more than 10 years, well controlled with medications. The patient had a history of tonic–clonic seizures 25 years prior, which were diagnosed as epilepsy after relevant investigations. The patient had no history suggestive of manifestations of SLE at that time. The initial treatment of phenytoin was stopped after 3 months owing to allergic rashes all over the body. The patient was switched to sodium valproate for the next 8 years. This was omitted by the treating neuro-physician after a prolonged remission. Fifteen years after the drug was stopped, the patient had a recurrence of convulsions. This time, the patient was started on tablet levetiracetam 500 mg three times a day after reconfirming that there was no other reason for the seizures.
The patient was asymptomatic for 1 year. Thereafter she started developing rashes all over her body, which increased gradually over the next 18 months. During this period the patient self-medicated and consulted her family physician, without a definitive diagnosis. Probably, steroid ointments were used. The patient consulted a dermatologist when the rashes worsened and were nonresponsive. The rash was characterised by well-defined erythematosus plaques. The rash was predominantly present on the face, neck and photo-exposed parts of chest. Lesions were absent on the left chest, an area usually covered by the patient’s clothing. Some plaques on the back had a halo of hypopigmentation, suggestive of topical steroid use. There were some healed lesions with hypopigmented scars (Figure 1). The lesions were non-itchy. There was no history of fever, joint pains or contact with allergens.
Figure 1 Lesions at presentation
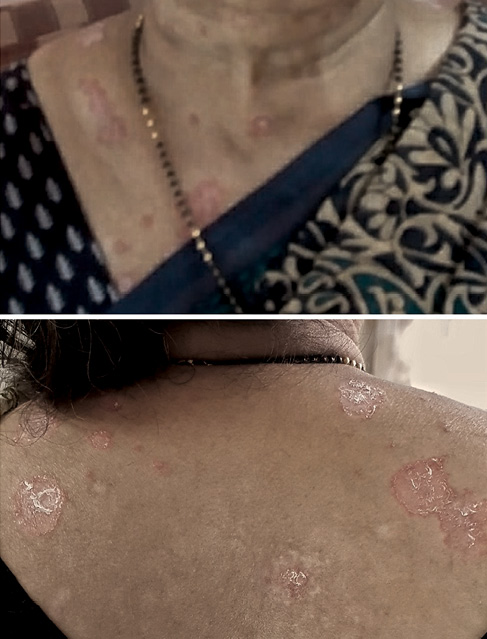
Based on the morphology, the lesions were diagnosed as SLE. The patient’s antinuclear antibody was detected to be 1 : 320, with a homogenous pattern. The patient was referred to us for further management.
On presentation, the patient was haemodynamically stable. Apart from the rashes, the systemic examination was unremarkable. The patient’s weight was 65 kg and body mass index was 25 kg/m2. Investigations revealed haemoglobin of 12 gm%, white cell count of 4,300 per cubic mm, platelet count of 140,000 per cubic mm, erythrocyte sedimentation rate of 72 mm at the end of 1 hour, serum creatinine of 0.9 mg% and normal liver enzymes. The antibodies to double-stranded deoxyribonucleic acid, histones and other nuclear antigens were negative. Complement levels were normal. Urine showed trace proteins but no sediments or casts.
The patient was diagnosed as SLE with prominent dermatological symptoms. Similar to other SLE patients, the patient was started on prednisolone 40 mg daily and hydroxychloroquine 200 mg twice a day. Fundoscopy was carried out prior to initiating hydroxychloroquine. Azathioprine 50 mg per day was increased to 100 mg per day after 1 month owing to paucity in response. Even with a relatively higher dose for predominantly dermatological symptoms, there was inadequate response after 3 months. Hence, azathioprine was substituted with mycophenolate mofetil 2 g per day. The patient responded partially with up to 50% reduction in lesions in the next 3 months. Since levetiracetam is considered a safe drug the diagnosis of DIL was missed initially.
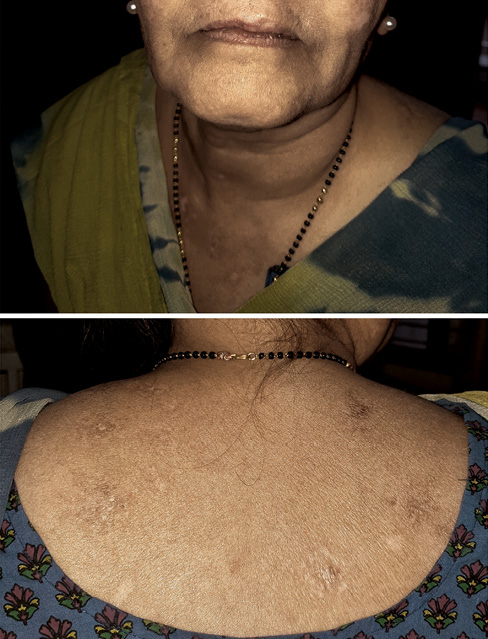
At this time it was realised that levetiracetam could be the cause of her SLE. Levetiracetam was stopped and substituted with sodium valproate, the medication the patient had tolerated well earlier. In less than 1 month, the lesions improved dramatically (Figure 2). The steroids were tapered and stopped after 3 months. The mycophenolate was tapered and stopped after 6 months. The patient was asymptomatic 3 months after stopping all medications. There were no episodes of seizures.
Figure 2 Lesions disappearing after treatment and withdrawal of levetiracetam
Discussion
In this patient, the typical morphology of lesions and strong antinuclear antibody positivity were strongly suggestive of SLE. The temporal relation between drug use and skin lesions suggested levetiracetam as the cause of the patient’s disease. The symptoms appeared within 1 year after starting levetiracetam. There were no immunological symptoms prior to starting levetiracetam and these vanished after the withdrawal of levetiracetam. There were only cutaneous manifestations in this patient. However, even one or more clinical symptoms can be suggestive of DIL.7 Antihistone antibodies, which are strongly associated with DIL, were negative. However, though these are positive in many patients with DIL, they have a varying prevalence according to the drug.8 A repeat challenge of the levetiracetam would have confirmed the causative relation of the levetiracetam, but this was considered unethical. Hence, though there are no criteria set for diagnosis of DIL, we can strongly suspect that this patient suffered with levetiracetam-induced SLE.
Rheumatologists should always be aware of drugs as the cause of SLE, especially in older patients. DIL is an autoimmune disorder caused by certain medications that present with features of SLE. By comparison, symptoms of DIL tend to be milder, present at later ages and tend to resolve after discontinuation of the drug.1 Symptoms may not start until the patient has been taking the drug for at least 1 month, however, it could take as long as 2 years.9 Males are as equally effected as females, and whites are more affected than blacks.9
Depending on the level of evidence seen from prospective studies, case series/reports or anecdotal reports,10 drugs causing SLE can be defined as definitive (isoniazid,1,2 procainamide,1,4 hydralazine1,3), probable (anticonvulsants such as phenytoin5 and carbamazepine6) and presumable (lithium and lamotrigine10). A fourth group of ‘recently reported drug’ are those that have increasing reports of lupus-like syndrome, such as etanercept and infliximab.11 Accumulating data will direct these drugs to their respective groups.
Levetiracetam is a broad-spectrum antiepileptic used for various types of epilepsies. It is well tolerated, and is considered a safe and effective antiepileptic. Common adverse effects include drowsiness, asthenia, headache and psychological symptoms. Dermatological adverse events with levetiracetam include urticarial vasculitis and leukocytoclastic vasculitis, presenting as erythematosus, annular and aciform urticarial wheals on the limbs.12 A study of 1,890 patients reported the presence of maculopapular skin lesions in approximately 0.6% of individuals with levetiracetam.13 However, currently there are no reports of SLE associated with levetiracetam. Slower acetylation may play a role in the greater predisposition of an elderly person, like this patient, in development of DIL.14 It is possible that the slow acetylation phenotype of this patient could be the mechanism of her DIL.
SLE is usually a disease of young females. Onset of SLE beyond 50–65 years is rare and seen in only 12–18% of SLE patients.15 This patient, a 62-year-old postmenopausal female, was beyond the typical age for presentation as SLE. This emphasizes the need to look for a predisposing cause in patients presenting with late-onset SLE.
This case report also stresses the need for a constant assessment of safety of newer drugs. Clinicians should keep DIL as a part of the differential diagnosis while assessing the dermatological side effects of levetiracetam.
Conclusion
Levetiracetam should be kept in mind as a cause of DIL. Secondly, in elderly onset SLE, drugs should be borne in mind as a cause of the disease. 
Acknowledgement
We thank Ms Sonal Ganore for her help in preparing the figures.
References
1 Vedove CD, Giglio MD, Schena D et al. Drug-induced lupus erythematosus. Arch Dermatol Res 2009; 301: 99–105.
2 Salazar-Paramo M, Rubin RL, Garcia-De La Torre I. Systemic lupus erythematosus induced by isoniazid. Ann Rheum Dis 1992; 51: 1085–7.
3 Nassberger L, Sjoholm AG, Jonsson H et al. Auto antibodies against neutrophil cytoplasm components in systemic lupus erythematosus and in hydralazine-induced lupus. Clin Exp Immunol 1990; 81: 380–3.
4 Blomgren SE, Condemi JJ, Vaughan JH. Procainamide-induced lupus erythematosus: clinical and laboratory observations. Am J Med 1972; 52: 338–48.
5 Singsen BH, Fishman L, Hanson V. Antinuclear antibodies and lupus-like syndromes in children receiving anticonvulsants. Pediatrics 1976; 57: 529–34.
6 Alballa S, Fritzler M, Davis P. A case of drug induced lupus due to carbamazepine. J Rheumatol 1987; 14: 599–600.
7 Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci 2007; 1108: 166–82.
8 Cozzani E, Drosera M, Gasparini G et al. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmune Dis 2014; 2014: 321–59.
9 Drug-induced lupus erythematosus 2018. https://emedicine.medscape.com (accessed 13/05/2020).
10 Ravindran V. Lamotrigine-induced lupus: a case report. Int J Rheum Dis 2011; 14: e47–8.
11 Ramos-Casals M, Brito-Zeron P, Soto MJ et al. Autoimmune disease induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol 2008; 22: 847–61.
12 Mangal SS, Kumaran S. Levetiracetam induced urticarial vasculitis: a preliminary report. Indian J Dermatol 2014; 59: 423.
13 Arif H, Buchsbaum R, Weintraub D et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology 2007; 68: 1701–9.
14 Rovensky J, Tuchynova A. Systemic lupus erythematosus in the elderly. Autoimmunity Rev 2008; 7: 235–9.
15 Yung RL, Richardson BC. Drug-induced lupus mechanisms. In: Lahita RG, editor. Systemic Lupus Erythematosus. 5th Edition. Oxford: Elsevier; 2011. pp. 385–403.