Introduction
Considerable recent advancements in tumour biology and the development of anticancer therapies have led to significantly improved survival and quality of life for patients with malignancy.1 Nevertheless, the trade-off for improved survival in this growing population is an increased incidence of major organ dysfunction in patients, including both acute kidney injury (AKI) and chronic kidney disease (CKD).
AKI in patients with cancer occurs most commonly in the context of either a pre-renal insult (secondary to volume depletion due to gastrointestinal fluid loss, impaired oral intake or hypercalcaemia) or obstructive uropathy (from abdominopelvic malignant disease, nephrolithiasis or retroperitoneal fibrosis secondary to radiotherapy). The development of a wide variety of intrinsic renal pathologies (glomerular disease, tubulointerstitial disease or thrombotic microangiopathy) not only as paraneoplastic phenomena but also as sequelae of traditional cytotoxic chemotherapy, novel targeted agents or immunotherapeutic agents are now increasingly well described.2 Systemic chemotherapeutic agents can cause acute tubular injury (platinum-based compounds, antimetabolite agents, ifosfamide, pemetrexed and methotrexate), thrombotic microangiopathy (gemcitabine, cisplatin and mitomycin C) or interstitial nephritis (ifosfamide and nitrosureas). Targeted anticancer and immunotherapeutic agents can also cause kidney disease secondary to acute tubular injury (inhibitors of BRAF, anaplastic lymphoma kinase (ALK), tyrosine kinase, cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), and rituximab), thrombotic microangiopathy (anti-vascular endothelial growth factor therapies, inhibitors of BRAF, ALK, tyrosine kinase, CTLA-4 and PD-1, and interferon), tubulointerstitial nephritis (inhibitors of BRAF, ALK, tyrosine kinase, CTLA-4 and PD-1), focal segmental glomerulosclerosis (tyrosine kinase inhibitors and interferon), minimal change disease (CTLA-4 and PD-1 inhibitors) or immune complex mediated glomerulonephritis (CTLA-4 and PD-1 inhibitors). Additionally, bisphosphonates are commonly given to cancer patients and can cause both acute tubular injury (pamidronate and zolendronate) and focal segmental glomerulosclerosis (pamidronate).
Paraneoplastic glomerular disease most commonly presents with the nephrotic syndrome. Membranous nephropathy is associated with solid organ tumours in 10–20% of cases and minimal change nephropathy is linked to Hodgkin’s lymphoma. Pauci-immune crescentic glomerulonephritis, with or without the presence of antineutrophil cytoplasmic antibodies (ANCA), has also been reported with malignancy.3 Renal cell carcinoma is the most common cancer associated with crescentic glomerulonephritis, and patients with ANCA-associated vasculitis have been shown to be eight times more likely to develop renal cell carcinoma compared to controls.4 The relationship between colorectal malignancy and crescentic glomerulonephritis is less well defined.
Case presentation
A 74-year-old male with a past medical history of hypertension, epilepsy and osteoarthritis was diagnosed with a poorly differentiated rectal adenocarcinoma that was treated surgically with an anterior resection. One out of 11 lymph nodes examined as part of the surgical specimen contained evidence of malignancy and so the patient proceeded to receive adjuvant cytotoxic chemotherapy with capecitabine. Other regular medications included amlodipine, sodium valproate and pro re nata ibuprofen. During the course of capecitabine treatment he developed intermittent diarrhoea and a painful blistering rash affecting his feet, which led to his treatment being curtailed following completion of six of the scheduled eight cycles of capecitabine. This was presumed to be secondary to ‘hand–foot’ syndrome, a common complication of capecitabine.5
Investigations performed following cessation of the capecitabine treatment revealed a normal serum creatinine of 77 µmol/L and a raised carcinoembryonic antigen level of 14 µg/L. Consequent magnetic resonance imaging revealed multiple metastatic lesions within the left lobe of the liver. The patient was planned to undergo a branch portal vein embolisation followed by partial hepatectomy; however, blood tests revealed an AKI with a serum creatinine of 364 µmol/L and significant proteinuria. Review of other investigations demonstrated an anaemia with a normal platelet count, normal coagulation parameters and a normal serum calcium (Table 1). Clinical assessment at this point revealed a blood pressure of 137/73 mmHg, pitting oedema to the sacrum and 3+ blood and 3+ protein on urinalysis.
Table 1 Selected laboratory investigations at time of development of acute kidney injury
|
|
|
|
|
|
|
White blood count
|
6.5 × 109/L
|
4–11 × 109/L
|
|
Haemoglobin
|
64 g/L
|
130–170 g/L
|
|
Platelet count
|
230 × 109/L
|
150–400 × 109/L
|
|
|
|
Prothrombin time
|
11.1 seconds
|
9–12 seconds
|
|
Fibrinogen
|
6.0 g/L
|
2–4 g/L
|
|
|
|
Sodium
|
138 mmol/L
|
133–146 mmol/L
|
|
Potassium
|
4.4 mmol/L
|
3.5–5.3 mmol/L
|
|
Urea
|
21.7 mmol/L
|
2.5–7.8 mmol/L
|
|
Creatinine
|
364 µmol/L
|
59–104 µmol/L
|
|
Bicarbonate
|
23 mmol/L
|
22–29 mmol/L
|
|
Adjusted calcium
|
2.32 mmol/L
|
2.2–2.6 mmol/L
|
|
Phosphate
|
1.58 mmol/L
|
0.8–1.5 mmol/L
|
|
Albumin
|
31 g/L
|
35–50 g/L
|
|
Bilirubin
|
4 µmol/L
|
0–21 µmol/L
|
|
Alkaline phosphatase
|
96 U/L
|
30–130 U/L
|
|
Alanine transaminase
|
14 U/L
|
7–40 U/L
|
|
Urine albumin: creatinine
|
167.6 mg/mmol
|
0–2.5 mg/mmol
|
|
|
|
Anti-nuclear antibody
|
0.1 units
|
0–0.9 units
|
|
Anti-neutrophil cytoplasmic antibody:
Proteinase 3
Myeloperoxidase
|
<0.7 IU/mL
<0.3 IU/mL
|
0–1.9 IU/mL
0–3.4 IU/mL
|
|
Anti-glomerular basement membrane antibody
|
1 IU/mL
|
0–7 IU/mL
|
|
Complement:
C3
C4
|
1.41 g/L
0.23 g/L
|
0.75–1.65 g/L
0.14–0.54 g/L
|
|
Serum electrophoresis
|
No paraprotein detected
|
|
|
Urinary electrophoresis
|
Negative
|
|
|
|
|
Hepatitis B surface antigen
|
Not detected
|
|
|
Hepatitis B core antibody
|
Not detected
|
|
|
Hepatitis C antibody
|
Not detected
|
|
|
HIV antigen/antibody
|
Not detected
|
|
| |
|
|
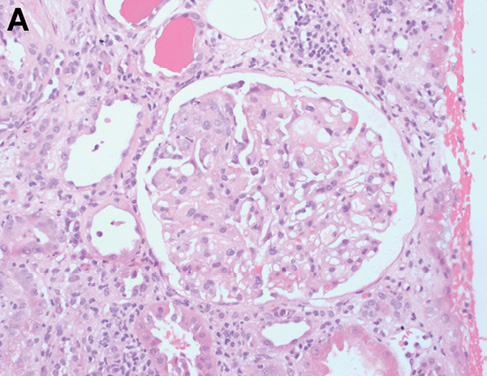
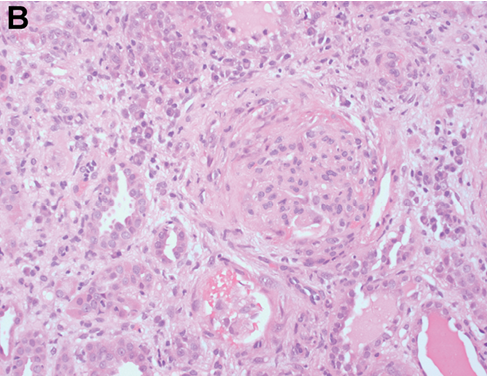
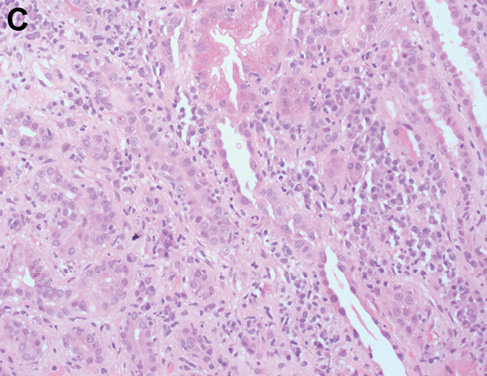
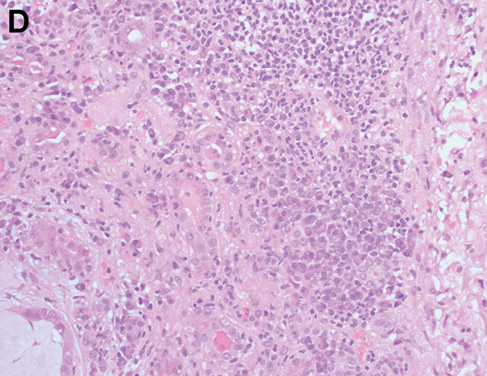
An intrinsic immunological and virological renal screen was unremarkable (Table 1). Renal tract imaging did not reveal any evidence of obstructive uropathy or renal vein thrombosis as a cause for the AKI. The patient proceeded to receive a blood transfusion prior to undergoing a renal biopsy, which identified a crescentic glomerulonephritis with endocapillary hypercellularity and active cellular crescents without immune deposits (Figure 1A,B). Active tubulointerstitial nephritis was also present (Figure 1C,D) which may have been related to ibuprofen use or, alternatively, the crescentic glomerulonephritis itself. The patient was treated initially with 1 mg/kg of oral prednisolone with appropriate gut, bone and antimicrobial prophylaxis. Prednisolone was subsequently weaned over the next two months with some improvement in his renal function, although he was still left with significant CKD (serum creatinine 187 µmol/L; estimated glomerular filtration rate 30 ml/min/1.73 m2). Given the ongoing impairment in renal function, further oncological treatment in the form of additional chemotherapy or surgery was deferred. Unfortunately, the patient was subsequently admitted with deteriorating renal and hepatic function. Despite active medical management with parenteral antibiotics to cover a presumed bacterial infection and intravenous fluids, the patient continued to deteriorate. He then received palliative care, following which he died.
Figure 1 Representative images showing glomeruli with endocapillary hypercellularity (a, b) and crescent formation (b); and tubulointerstitial nephritis, which is plasma cell-rich with areas of lymphocytic tubulitis (c, d). Haematoxylin and eosin-stained sections at ×200 magnification.
Discussion
The relationship between malignancy and a wide variety of renal diseases is well established, although the exact pathogenesis of crescentic glomerulonephritis in patients with malignancy is unclear. Proteinuria in the setting of malignancy has been more extensively studied and carcinoembryonic antigen–antibody complexes have been identified in a patient with colonic malignancy and nephrotic syndrome.6 Additional animal studies identified that rats with metastatic growth of RCN-9 cells in the liver developed significant proteinuria with glomerular immunoglobulin G deposition seen on immunohistochemistry and electron-dense deposit and podocyte foot process effacement observed on electron microscopy.7
The development of both metastatic malignancy and autoimmune disease are characterised by complex functional and compositional changes to the immune system. Malignancy induces proliferation of bone marrow haematopoietic stem cells, multipotent progenitors and granulocyte monocyte progenitors that can contribute to altered systemic immunity and subsequent disease progression.8 A greater understanding of these changes in the context of malignancy has led to the increasing use of immunotherapeutic agents to manipulate the immune response against malignant tissue, at the risk of the development of autoimmune diseases that can affect a wide variety of organs, including the kidney.
Kidney impairment has been shown to be associated with worse prognosis in patients with cancer. Two large cohort studies from South Korea and Taiwan, involving 8,223 and 123,717 adults respectively, revealed an adjusted hazard ratio for overall cancer mortality between 1.12 and 1.75 for patients with CKD, with worse outcomes for patients with more advanced stages of CKD.9,10
Conclusion
This case highlights the deleterious impact of kidney impairment on patients with malignancy. The development of significant kidney impairment meant that this patient was no longer a suitable candidate for surgical treatment of his liver metastases and was unable to receive additional cytotoxic chemotherapy. During his final admission he developed worsening liver function tests, which may have been related to progression of his liver metastases, and this may have contributed to his poor outcome.
Despite recent advances in cancer therapeutics, the co-management of both active malignancy and kidney impairment remains an evolving challenge for both oncologists and nephrologists. The key priorities for research and multidisciplinary working to improve the outlook for this subgroup of patients have been highlighted recently at an international consensus meeting.11
