Introduction
Tuberculosis (TB) continues to be a major infectious disease worldwide. TB is among the top ten causes of death and the leading cause of death from a single infectious agent surpassing HIV/AIDS. Based on the 2020 World Health Organization (WHO) Global Tuberculosis Report, approximately 10 million people contracted the disease, resulting in 1.4 million deaths worldwide.1 The burden of disease is particularly high in developing countries, with 25% of TB occurring in India.1,2 Extrapulmonary tuberculosis (EPTB) including intestinal TB (ITB) can occur in isolation or in conjunction with disseminated TB. Nearly 50% of cases of EPTB occur in HIV patients while 15% to 20% of cases are seen in immunocompetent patients.3 One tenth of EPTB cases have abdominal involvement, with approximately 5% affecting the gastrointestinal tract and peritoneum.4 The ileocecal region forms the favourite site of ITB and the less frequent sites include ascending colon appendix, sigmoid colon, rectum, jejunum, stomach and oesophagus.3
The diagnosis of ITB can be challenging due to nonspecific symptoms, atypical presentations, difficulty in tissue acquisition for histopathology and the low yield of microorganism in culture. In addition, the histopathological features and imaging characteristics from radiological studies often mimic inflammatory bowel disease, especially Crohn’s disease (CD).
An accurate diagnosis of ITB is of paramount importance as it can be cured using anti-tuberculous treatment (ATT). On the other hand, mistaking ITB for CD and initiating treatment with immunomodulators and biologics can have devastating consequences.
There are no established national or international guidelines/protocols in the work up/diagnosis of gastrointestinal tuberculosis. Standard TB cultures can take two to six weeks for mycobacterium tuberculosis complex (MTBC) to grow, and conventional drug resistance tests can add three more weeks, delaying institution of treatment. INDEX TB guidelines recommends the use of Xpert MTB/RIF, a rapid diagnostic test for detecting MTBC and resistance to rifampicin in less than two hours as a diagnostic tool for EPTB.5
With this background, our pilot study utilised a combination of diagnostic modalities including colonoscopy with biopsy for histopathology, Xpert MTB/RIF and TB culture to arrive at the best sensitivity and specificity. We also employed favourable outcome with ATT as a benchmark for ascertaining the accuracy in diagnosis of ITB with a two-year follow up of study participants.
Methods
Patient population and study design
We conducted a retrospective, observational cohort study of 426 patients (adults and children) who presented to the Department of Digestive Diseases between January 2016 and December 2018, with clinical suspicion of ITB or had a diagnostic confusion between ITB or CD. All patients were evaluated by colonoscopy and tissue samples were taken for histopathology, Xpert MTB/RIF and TB culture. The following were excluded from the study: those who had laparoscopic omental/peritoneal biopsies as diagnostic material and those who had less than a two-year follow up.
Clinical data
Patient demographics (age, gender), presenting symptoms, past history/family history of TB, colonoscopy/ileoscopy with biopsy, histopathology, Xpert MTB/RIF and TB culture results were obtained from the hospital information system. Patients who had colonoscopy findings and supportive histological features consistent with ITB or positive Xpert MTB/RIF or positive TB culture were started on ATT for six months. Follow-up data were collected during clinic visits at one month, two months, three months, six months, one year and two years. If the clinical response to ATT was unfavourable at one to two months, ATT was stopped, and additional investigations were done for alternate diagnosis.
Definitions
Although the gold standard for diagnosing TB is culture, it is time consuming (three to eight weeks) and has low sensitivity,3 attributed largely due to the paucibacillary nature of the disease or due to prior treatment with ATT. Therefore a composite reference standard (CRS) was used for benchmarking to ensure all cases including those not detected by culture were evaluated with clinical outcome at the end of the two-year follow up. CRS in this study refers to the diagnosis of ITB being made in the appropriate clinical setting with colonoscopic features supported by either positive TB culture or positive Xpert MTB/RIF or histological features of intestinal TB (tubercular granuloma) or AFB positivity on Zeil Nelson ZN special stain, together with favourable outcome with ATT evidenced by mucosal healing at the end of therapy and good clinical response with no recurrence of symptoms for at least one year after completion of therapy.
CRS could be applied in our retrospective observational study, as it is the standard practice to take tissue samples at the time of colonoscopy for histopathology, Xpert MTB/RIF and culture in all clinically suspected cases. CRS has been previously used by other authors.3,6
Colonoscopy examination
Colonoscopy examination was done with a video colonoscope (Olympus EVIS EXERA III CF-H 190 series, Tokyo, Japan) after bowel preparation with polyethylene glycol. Colonoscopic parameters for suspicion of ITB were a combination of three or more of the following characteristics (i) isolated ileal or ileocecal involvement/<4 segment involvement (ii) ileocecal valve destruction (iii) circumferential ulcers (iv) short-segment strictures (v) mucosal nodularity in colon (v) pseudo-polyps. Multiple segmental colonic biopsies were taken for histopathological examination, Xpert MTB/RIF and TB culture. In those patients who received ATT, a colonoscopy was performed at the end of therapy to assess for mucosal healing.
Histopathological examination
The typical histological parameters for the diagnosis of ITB included ileocecal ulceration with ulcers lined by epithelioid histiocytes, presence of granuloma (necrotising medium to large, discreet/confluent, multiple, deep mucosal/submucosal granulomata), and possible demonstration of acid-fast bacilli (AFB) by modified Ziehl Neelsen (ZN) stain. Those patients with colonoscopic and histological changes confined to the ileocecal valve with presence of medium sized non-necrotising granulomata were also considered eligible for therapeutic trial of ATT.
Xpert MTB/RIF
One ml of homogenised samples was processed according to standard protocol recommended by WHO6a. The samples were mixed with 2ml of Xpert MTB/RIF sample reagent and finally 2ml was transferred to Xpert MTB/RIF cartridge (Cepheid Inc., Sunnyvale, CA, USA) and loaded into the machine. In the machine, DNA extraction and amplification of a 192-bp segment of the rpoB gene occurs by real time PCR, and the results are generated in 1 hour 52 minutes. Detection is achieved by hybridisation of the amplified products with five overlapping probes complementary to the rpoB ‘core’ region (81 bp), determining rifampicin resistance.
TB culture
Cultures were done in the automated BACTEC MGIT 960 instrument (Becton Dickinson, Sparks, Md.). All clinical specimens were digested and decontaminated and inoculated into MGIT 960 vials as described by the manufacturer, and 0.2ml onto Lowenstein Jensen (LJ) medium slants. MGIT vials were incubated until they are flagged positive by the instrument or for a maximum of six weeks. LJ medium slants were examined daily for one week and thereafter, once a week for ten weeks, for the visible appearance of colonies. All positive MGIT vials or LJ slants were confirmed for AFB by staining and further subjected to identification of MTBC, by the MPT64 protein detection-based immunochomatographic test (SD Bioline Kit, Standard Diagnostics, Inc., Korea).
Treatment and follow up
All diagnosed patients were given six months ATT as recommended by INDEX TB guidelines;5 induction regimen for two months (isoniazide 5mg/kg, rifampicin 10mg/kg, pyrazinamide 20-25mg/kg and ethambutol 15-20mg/kg), followed by maintenance therapy of isoniazide, rifampicin and ethambutol for four months. Clinical response to ATT was assessed at the end of one month, two months, three months, and responders continued ATT for six months and re-evaluated at the end of treatment by colonoscopy for mucosal healing or resolution of symptoms on follow up. All patients were followed up at one year and two years to assess if there was any reappearance of symptoms. Therapeutic trial of ATT was given to patients in whom there was a strong clinical and colonoscopic suspicion, even in the absence of supportive results from ancillary tests (histology, Xpert MTB/RIF and culture).
Statistical analysis
A McNemar test was used to compare the various diagnostic modalities with CRS (follow-up outcome). Statistical analyses were conducted using SPSS Version 20.0 for Windows (IBM Corporation ARMONK, NY, USA). Validity parameters such as sensitivity, specificity, accuracy, positive predictive values (PPV) and negative predictive values (NPV) of histopathology and Xpert MTB/RIF, culture with CRS taken as the gold standard.
Ethical approval
The pilot study was conducted with the approval from the institutional ethics committee (IRB No. AIMS 2018-158, dated 20-06-2018). An informed consent was obtained from all study participants.
Results
A total of 426 patients were included into the study, all of whom had colonoscopy with mucosal biopsies taken for histopathology, Xpert MTB/RIF and TB microbiology culture studies and in whom a two-year follow up was available to assess clinical outcome. ITB was diagnosed in 35 (8.2%) of patients, all of whom completely recovered with ATT. Additionally, there were 78 (18.3%) patients with CD, nine patients (2%) with ulcerative colitis and the remaining 305 (71.6%) patients had other non-related conditions such as hypertension, diabetes mellitus, chronic kidney disease, arthritis, malignancies like carcinoma colon, etc. (Table 1).
Table 1 Patient demographics, results of diagnostic modalities and anti-tuberculous therapy
|
|
|
|
Age (years)
|
43.0 ± 17.5
|
|
Gender
Female
Male
|
166 (39.0)
260 (61.0)
|
|
Diagnosis
ITB
CD
Others
UC
|
35 (8.0)
78 (18.3)
305 (71.6)
9 (2.1)
|
|
Diagnostic modality
|
|
|
Colonoscopy
Negative
Positive
|
327 (76.8)
99 (23.2)
|
|
Histopathology
Negative
Positive
|
388 (91.1)
38 (8.9)
|
|
Xpert MTC/RIF
Negative
Positive
|
412 (96.7)
14 (3.3)
|
|
TB culture
Negative
Positive
|
393 (92.3)
33 (7.7)
|
|
Treatment
|
|
|
Anti TB therapy
Not given
Given
|
376 (88.3)
50 (11.7)
|
ITB: intestinal tuberculosis; CD: Crohn’s disease; UC: ulcerative colitis and anti-tuberculous therapy
Patient characteristics
There was a wide age group of distribution (range 5-82 years), mean age of presentation was 43 years and with a slight male preponderance (Table 1). A past history and family history of pulmonary or EPTB was obtained in 9.24% and 2.4% patients, respectively. None of the patients in this series was co-infected with HIV.
Colonoscopic findings
117 patients had colonoscopic abnormalities, of which 99 patients were suspected with ITB, 30 patients demonstrated typical morphological features accepted for ITB. Colonoscopy missed the diagnosis of ITB in five patients, all of whom had CD. The colonoscopic findings were mostly seen as ileocecal/right colon ulceration and nodularity. The significant p-value is due to the high false positivity.
Histopathological findings
32 patients who were histologically diagnosed as ITB had necrotising granulomata, which were discreet/confluent, deep seated in the mucosa/submucosa with variable degree of lymphoid cuffing (tubercular granuloma) (Figure 1). Six patients with mucosal ulceration and medium sized non-necrotising granulomata confined to the ileocecal valve were also given a trial of ATT despite the lack of supportive evidence from Xpert MTB/RIF and TB culture, but evolved as CD on follow up. The accuracy of the histological diagnosis was 97.9% (Table 2). The p-value was not found to be significant due to high numbers of true positive and negatives. Interestingly, ITB was histologically diagnosed in a patient with CD and later confirmed by positive TB culture. Only one patient demonstrated AFB positivity on ZN stain.
Figure 1a Colonic mucosa showing ulceration, increased lamina propria inflammatory cells with crypt architectural distortion and two deep seated granuloma (marked) with lymphoid cuffing in the submucosa (H&E x40). Figure 1b Large epithelioid granuloma with central necrosis (tubercular granuloma) (x200).
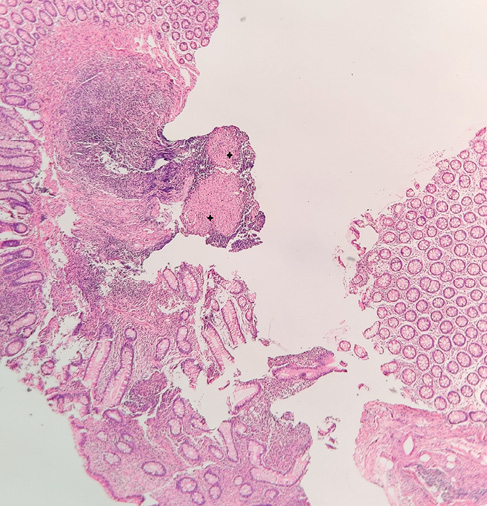
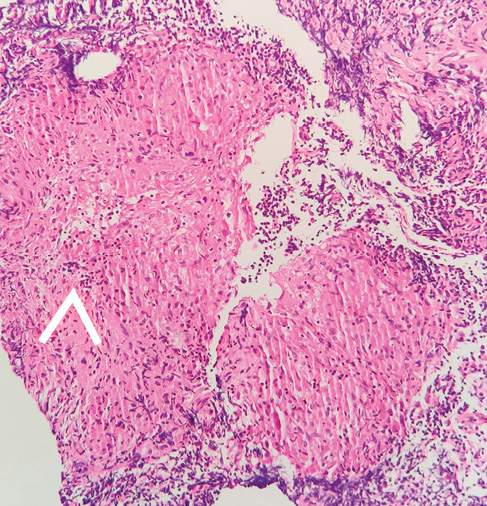
Table 2 Comparison of the performance of the various diagnostic modalities used in ITB, using CRS as gold standard
|
Diagnosis modality
|
Result
|
Negative
n (%)
|
Positive
n (%)
|
P-value
|
Sensitivity
|
Specificity
|
PPV
|
NPV
|
Accuracy
|
|
Colonoscopy
|
Negative
Positive
|
322 (98.5)
69 (69.7)
|
5 (1.5)
30 (30.3)
|
<0.001
|
85.7
|
82.4
|
30.3
|
98.5
|
82.6
|
|
Histology
|
Negative
Positive
|
385 (99.2)
6 (15.8)
|
3 (0.8)
32 (84.2)
|
0.508
|
91.4
|
98.5
|
84.2
|
99.2
|
97.9
|
|
Xpert
|
Negative
Positive
|
391 (94.9)
–
|
21 (5.1)
14 (100.0)
|
<0.001
|
40.0
|
100.0
|
100.0
|
94.9
|
95.1
|
|
TB culture
|
Negative
Positive
|
391 (99.5)
–
|
2 (0.5)
33 (100.0)
|
0.500
|
94.3
|
100.0
|
100.0
|
99.5
|
99.5
|
|
Histology+Xpert
|
Negative
Positive
|
385 (99.7)
6 (15.0)
|
1 (0.3)
34 (85.0)
|
0.125
|
97.1
|
98.5
|
85.0
|
99.7
|
98.4
|
|
Histology+Xpert
+Colonoscopy
|
Negative
Positive
|
322 (100.0)
69 (66.3)
|
–
35 (33.7)
|
<0.001
|
100.0
|
82.4
|
33.7
|
100.0
|
83.8
|
|
Histology+Xpert
+Culture
|
Negative
Positive
|
385 (100.0)
6 (14.6)
|
–
35 (85.4)
|
0.031
|
100.0
|
98.5
|
85.4
|
100.0
|
98.6
|
|
ITB: intestinal tuberculosis; CRS: composite reference standard. in this study CRS refers to the diagnosis of ITB being made in the appropriate clinical setting with colonoscopic features supported by either positive TB culture or positive XpertMTB/RIF or histological features of intestinal TB (tubercular granuloma) or AFB positivity on Zeil Nelson special stain, mandated by favourable outcome with ATT as evaluated by clinical response and colonoscopic evidence of mucosal healing at the end of therapy/follow up.
|
Xpert MTB/RIF
Xpert MTB/RIF was detected in 14 (3.3%) patients, all of whom had positive TB culture. Of the 14 cases, only two were without definitive supporting histopathological features. Though the sensitivity of Xpert MTB/RIF was low, specificity and PPV was 100%, with an accuracy of 95.1% (p-value <0.001) (Table 2). None of the 14 patients showed resistance to rifampicin by Xpert MTB/RIF.
TB culture
33 patients had positive growth on culture. An additional two patients were detected using the CRS (Table 2).
Combinative evaluation of diagnostic modalities
On evaluating the three diagnostic modalities (colonoscopy, histopathology, Xpert MTB/RIF), it was found that histopathology had the highest sensitivity (91.4%) and NPV (99.2%), MTB/RIF had the highest specificity (100%) and PPV (100%). A combination of the various modalities improved sensitivity and specificity. The best diagnostic yield with highest sensitivity and specificity was noted when TB culture was combined with histopathology and Xpert MTB/RIF (Table 2).
ATT therapy
ATT was given to all 35 patients diagnosed as ITB based on histopathology/Xpert MTB/RIF and/or culture and clinical response was evaluated at one, three and six months and colonoscopy for mucosal healing or resolution of symptoms at the end of therapy/follow up. Six patients with clinical suspicion, deep ulceration of the IC valve and medium-sized granuloma (non-necrotising), unsupported by Xpert and/or TB culture positivity, were given a trial of ATT, but without favourable response and evolved into CD. There were no patients who showed response to empirical ATT therapy in the absence of positive results from supportive ancillary investigative modalities (histology or Xpert MTB/RIF or culture).
Discussion
Tuberculosis is endemic in developing countries and a small but significant number of such patients will have ITB. The diagnosis of ITB continues to be a challenge for clinicians and pathologists alike, especially differentiating ITB from CD. Both ITB and CD share common clinical, radiological and histological features. Accurate and early diagnosis of ITB is extremely important as specific treatment can often lead to a cure and a favourable outcome. If the diagnosis is not clear, it is often a practice in developing countries where TB is endemic to start empirical treatment with ATT. However, empirical treatment with ATT is not advisable since ATT is notorious in causing liver injury and, in many cases, leads to liver failure. Also, empirical treatment with ATT in a doubtful case, where the actual diagnosis is CD, will cause disease progression and loss of valuable time in treating CD. On the other hand, starting immunomodulators and biologics with a misdiagnosis of CD can result in a flare of TB and a bad outcome. Hence it is very important that an accurate diagnosis of ITB or CD is made before starting treatment.
With this background information, several diagnostic investigations have been introduced. The advent of colonoscopy provided a unique opportunity to directly visualise the colon and terminal ileum for identifying characteristic morphological lesions in addition to tissue acquisition for histopathology and Xpert/RIF and culture.3,7,8
In this study, a combination of colonoscopy with careful evaluation of the morphology of lesions, biopsy for histopathology, Xpert/RIF and culture were employed. It is well recognised that the clinical response to ATT is a crucial assessment tool to confirm ITB vs CD, as various diagnostic modalities (clinical, endoscopic, imaging, serological and histopathology) do not categorically differentiate between these two diseases. Hence, in this study, a favourable outcome with ATT was a mandated requisite in the CRS to assess the accuracy of the diagnosis.
The mean age of presentation in our study was 42 (5-82 years) and the male to female ratio was 1.56, similar to other studies.9,10 Abdominal pain, altered bowel habit, weight loss, and anaemia were the common clinical features as previously reported,11,12 all of which overlap with CD. Limsrivilai et al. in their meta-analysis found fever, night sweats, lung involvement and ascites favoured ITB.13 The presentation can also be as pseudotumor with intestinal obstruction.14 The diagnosis of ITB is challenging when patients present with little or no symptoms,15 or rarely when patients with CD develop ITB, as seen in one of our patients.
Colonoscopic findings of transverse ulcers, patulous IC valve, caecal involvement, less than four segments involvement and pseudo polyps are well described in ITB. However, similar findings can be present in CD. Various models integrating clinical and endoscopy with scoring systems have been proposed for differentiating between CD and ITB.11,16 These predictive models are still lacking in validation and practical application in a routine clinical setting. Li et al. in their study of 122 ITB and 130 CD patients used the above endoscopic parameters and obtained a sensitivity and specificity of 82.9% and 82.0%, respectively.17 Our study highlights the high rate of false positivity when relying on colonoscopy and symptoms alone, and therefore advocates the use of ancillary tests for ITB to establish the diagnosis. Follow-up colonoscopy is necessary for assessing resolution of mucosal injury. Mucosal assessment has been recommended as early as two months from starting therapy.18 Anti-mycobacterial therapy in patients with CD can show symptomatic response to ATT but predisposes to strictures. In our study, even if there were CD patients who responded initially to ATT, it is very unlikely that they remained asymptomatic in the two-year follow up.
Chronic granulomatous inflammation is seen in both ITB and CD, and the histological diagnosis can be challenging. Pulimood et al. suggested features such as ulcers lined by epithelioid histiocytes, tubercular granuloma (size >200microns, multiple >5-10/hpf, discreet and confluent, deep mucosal/submucosal location, lymphoid cuffing and caseating necrosis) favour TB over CD,19 which were employed in our study to make the diagnosis of ITB. Diagnostic accuracy improves with increased biopsies.20 In our study, histopathology had the highest sensitivity (91.4%) and NPV (99.2%). A trial of ATT is recommended when there are indeterminant features between ITB and CD, to avoid fatal complications of immunosuppressive therapy in a patient with ITB. Recent Asia-Pacific consensus suggest that in the dilemma between TB and CD, the latter disease should be considered in a patient who does not respond to ATT.21 In this context, supportive positive findings from Xpert MTB/RIF and TB culture will help to favour ITB over CD as seen in our study and assist in appropriate management, limiting the use of empirical ATT. The sensitivity of AFB with ZN stain is very low in ITB due to the paucibacillary nature of the disease. As per studies from India and S. Korea, MDR-TB has been found to be of low prevalence (<5%) in patients with ITB.22,23
Xpert MTB/RIF is a fully automated real-time PCR assay for detection of MTBC. The assay takes two hours for diagnosing TB in clinical samples. A positive result can be considered a definitive for diagnosis of TB. It significantly shortens the time for diagnosis which is four days for histopathology and six weeks for TB culture. Based on the positive report, treatment can be initiated immediately. Our study, along with other authors,3,6,24 have shown Xpert MTB/RIF extremely useful in confirming EPTB, with high specificity. Kumar et al.,3 Bellam et al.,25 Sharma et al.26 and our study have found low sensitivity of 8.1%, 32%, 23% and 40% respectively, but consistently showed high specificity (100%) and PPV (100%). The availability of test results within 12 hours makes it is a useful diagnostic tool especially in endemic areas, as also observed by Kumar et al.3
Historically, the gold standard for diagnosing TB is culture. Unfortunately, it is time consuming (3-8 weeks), and results are frequently negative,26 attributed largely due to the paucibacillary nature of the disease or due to prior treatment with ATT. The sensitivity of AFB culture has been variably reported as 2-40% using LJ medium.3,27,28 However, studies have shown that liquid culture system like BACTEC have a better yield when compared to solid cultures like LJ medium. The sensitivity of TB culture in our study was 94.3%, which is higher than previous studies by Leung et al., Shah et al. and Kirsch et al., who have reported sensitivities of 73%, 76% and 78%, respectively.29
In this study, 8.2% patients were identified as ITB with good response to ATT, 91.4% of whom had typical histological features. Considering the utility of the above-mentioned diagnostic tests, we found that a combinatorial approach that includes Xpert MTB/RIF with histopathology has optimum diagnostic value, and the specificity increased when TB culture was also included. The advantage of Xpert MTB/RIF is the diagnosis is obtained within 12 hours, as in 40% of our patients. Interestingly, Xpert MTB/RIF identified 5.7% (2/35) cases not positively ascertained by histopathology. By combining Xpert MTB/RIF with histopathology, 97% (34/35) of the ITB cases can be diagnosed within three days. With a specificity of 100%, Xpert MTB/RIF positive cases need not wait for confirmation by histopathology or culture.
Developing models integrating the above diagnostic modalities will significantly increase the accuracy of the diagnosis and reduce therapeutic trial of ATT. Once validated in larger robust studies, we hope that this model of combined use of ancillary testing (histology, Xpert MTB/RIF and TB culture if available) for diagnosis of ITB, epecially in endemic areas would be useful. The combinatorial approach may help to reduce the number of empirical ATT in patients with CD and limit drug toxicity.
Apart from a small sample size, lack of colonoscopic evaluation as early as two months of ATT to demonstrate early mucosal response6,18 is a major limitation of the present study.
The diagnosis of ITB can be challenging and requires a combinatorial diagnostic approach with clinical, colonoscopic, pathological features, and supportive microbiological studies. In this pilot study, using the diagnostic algorithm that included Xpert MTB/RIF, we could diagnose 40% cases on the same day while 97% cases could be identified within three days when combined with histopathology. Though appropriate validation is very much necessary, this approach appears to have relevance to the clinical practice especially in TB endemic areas. 
Acknowledgements
The authors are grateful to Mrs. Deepthy Divakaran, Tutor Physician Assistant, Department of Gastroenterology, Amrita Institute of Medical Sciences for all the assistance in obtaining the follow up (including telephonic contact) of the patients in the study.
References
1 World Health Organization. Global Tuberculosis Report 2020: executive summary. Geneva: World Health Organization; 2020.
2 Reid MJA, Arinaminpathy N, Bloom A et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 2019; 393: 1331-84.
3 Kumar S, Bopanna S, Kedia S et al. Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intest Res 2017; 15: 187-94.
4 Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res 2004; 120: 305-15.
5 Sharma SK, Ryan H, Khaparde S et al. Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J Med Res 2017; 145: 448-63.
6 Pratap Mouli V, Munot K, Ananthakrishnan A et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn’s disease. Aliment Pharmacol Ther 2017; 45: 27-36.
7 Mehta V, Desai D, Abraham P et al. Making a positive diagnosis of intestinal tuberculosis with the aid of new biologic and histologic features: how far have we reached? Inflamm Intest Dis 2019; 3: 155-60.
8 Makharia GK, Srivastava S, Das P et al. Clinical, endoscopic, and histological differentiations between Crohn’s disease and intestinal tuberculosis. Am J Gastroenterol 2010; 105: 642-51.
9 Jain M, Baijal R, Kumar P et al. Profile of patients with gastrointestinal TB at a tertiary care centre in western India. Trop Doct 2011; 41: 242-3.
10 Kentley J, Ooi JL, Potter J et al. Intestinal tuberculosis: a diagnostic challenge. Trop Med Int Health 2017; 22: 994-9.
11 Kedia S, Das P, Madhusudhan KS et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J Gastroenterol 2019; 25: 418-32.
12 Makharia GK, Srivastava S, Das P et al. Clinical, endoscopic, and histological differentiations between Crohn’s disease and intestinal tuberculosis. Am J Gastroenterol 2010; 105: 642-51.
13 Limsrivilai J, Shreiner AB, Pongpaibul A et al. Meta-analytic Bayesian model for differentiating intestinal tuberculosis from Crohn’s disease. Am J Gastroenterol 2017; 112: 415-27.
14 Androulaki A, Papathomas TG, Liapis G et al. Inflammatory pseudotumor associated with Mycobacterium tuberculosis infection. Int J Infect Dis 2008; 12: 607-10.
15 Sato S, Yao K, Yao T et al. Colonoscopy in the diagnosis of intestinal tuberculosis in asymptomatic patients. Gastrointest Endosc 2004; 59: 362-8.
16 Limsrivilai J, Pausawasdi N. Intestinal tuberculosis or Crohn’s disease: a review of the diagnostic models designed to differentiate between these two gastrointestinal diseases. Intest Res 2021; 22: 19: 21-32.
17 Li X, Liu X, Zou Y et al. Predictors of clinical and endoscopic findings in differentiating Crohn’s disease from intestinal tuberculosis. Dig Dis Sci 2011; 56: 188-96.
18 Sharma V, Mandavdhare HS, Dutta U. Letter: mucosal response in discriminating intestinal tuberculosis from Crohn’s disease-when to look for it? Aliment Pharmacol Ther 2018; 47: 859-860.
19 Pulimood A, Ramakrishna B, Kurian G et al. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn’s disease from tuberculosis. Gut 1999; 45: 537-41.
20 Mehta V, Desai D, Abraham P et al. Do additional colonoscopic biopsies increase the yield of Mycobacterium tuberculosis culture in suspected ileo-colonic tuberculosis? Indian J Gastroenterol 2018; 37: 226-30.
21 Ooi CJ, Makharia GK, Hilmi I et al. Asia Pacific Association of Gastroenterology (APAGE) Working Group on Inflammatory Bowel Disease. Asia Pacific consensus statements on Crohn’s disease. Part 1: definition, diagnosis, and epidemiology: Asia Pacific Crohn’s disease consensus – part 1). J Gastroenterol Hepatol 2016; 31: 45-55.
22 Samant H, Desai D, Abraham P et al. Acid-fast bacilli culture positivity and drug resistance in abdominal tuberculosis in Mumbai, India. Indian J Gastroenterol 2014; 33: 414-9.
23 Ye BD, Yang SK, Kim D et al. Diagnostic sensitivity of culture and drug resistance patterns in Korean patients with intestinal tuberculosis. Int J Tuberc Lung Dis 2012; 16: 799-804.
24 Kohli M, Schiller I, Dendukuri N et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev 2018; 8: CD012768.
25 Bellam BL, Mandavdhare HS, Sharma K et al. Utility of tissue Xpert-Mtb/RIF for the diagnosis of intestinal tuberculosis in patients with ileocolonic ulcers. Ther Adv Infect Dis 2019; 6: 2049936119863939.
26 Sharma V, Soni H, Kumar-M P et al. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 2021; 19; 253-65.
27 Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn’s disease in India where tuberculosis is widely prevalent. World J Gastroenterol 2008; 14: 7416.
28 Shah SR, Shenai S, Desai DC et al. Comparison of Mycobacterium tuberculosis culture using liquid culture medium and Lowenstein Jensen medium in abdominal tuberculosis. Indian J Gastroenterol 2010; 29: 237-9.
29 Mehta V, Desai D, Abraham P et al. Making a positive diagnosis of intestinal tuberculosis with the aid of new biologic and histologic features: how far have we reached? Inflamm Intest Dis 2019; 3: 155-60.
