Introduction
At any one time, nearly 30% of patients in acute hospitals in Scotland are in the last year of life.1 During that final year, there is evidence that between 30% and 40% of major medical interventions are likely to be non-beneficial,2,3 and this includes intravenous antimicrobials.
In addition to the problem of futility, treatments in end-of-life patients pose the risk of harm. In the case of antimicrobials, this extends beyond the treated individual to others who may subsequently acquire a drug-resistant organism. In 2017, Professor Dame Sally Davies, the Chief Medical Officer for England, stated: ‘The world is facing an antibiotic apocalypse. Unless action is taken to halt the practices that have allowed antimicrobial resistance to spread … we could return to the days when routine operations, simple wounds or straightforward infections could pose real threats to life’.4,5
Among terminally ill patients, the issue of antimicrobial prescribing is particularly problematic.6–9 Although a patient’s illness trajectory may be driven by advanced malignancy or irreversible organ failure, their final illness may indeed be due to infection, e.g. urinary or respiratory. Antimicrobial treatment is then prescribed despite the likelihood of futility. Clinicians sometimes justify treatment in these circumstances: the systemic effects of infection mediated by inflammatory cytokines, e.g. fever, can be palliated by antimicrobials.7,10,11 On the other hand, treating infection that occurs during terminal illness sometimes stalls the dying process. Further, even when death is imminent and continuing to treat is futile, there is often reluctance by clinicians to withdraw antimicrobial treatment.12
These complex issues are potentially addressed if a patient’s illness trajectory is recognised (‘diagnosing dying’), there is discussion about appropriate goals of care and a treatment plan is put in place. We have recently shown that a treatment escalation/limitation plan (TELP) results in a significant reduction in harms in patients at the end of life (Appendix 1).13
The aim of the present audit was to evaluate the prescribing pattern for antimicrobial therapy in terminally ill patients in University Hospital Wishaw (UHW), Wishaw, UK, and whether it was influenced by the use of a TELP.
Methods
The audit comprised a retrospective cross-sectional review of the hospital records for consecutive patients who died in the medical wards, High Dependency Unit and Coronary Care Unit in UHW between 1 May and 31 July 2018.
Data were obtained from each patient’s hospital notes, prescription records and laboratory results and were entered into a standardised pro forma, which was revised following a pilot evaluation. Data from the pilot were not included in the analyses.
Definitions
- The Gold Standards Framework Proactive Identification Guidance (GSF PIG) criteria were used to identify ‘expected’ and ‘unexpected’ deaths based on admission diagnosis and preadmission comorbidities.14 Categorisation was unrelated to, and distinct from, attending clinicians’ identification of an imminent death: ‘imminent’ or ‘inevitable’ meant that death was likely within hours or days.
- Antimicrobial prescribing was deemed to be ‘inappropriate’ if one or more of the following three criteria applied: 1) patients had a TELP with an antimicrobial ‘ceiling’; 2) the patient’s death was recognised to be imminent/inevitable; or, 3) when infection did not contribute to or cause death (based on death certificate data) and/or there was no direct laboratory evidence to indicate infection.
- Direct evidence of infection was defined as a positive culture result during the final admission. Indirect evidence was defined as having raised inflammatory markers, e.g. C-reactive protein.
- An adverse event was identified from patient notes and was dependent on having been recorded. Its severity was based on the perceived impact on the patient as assessed by the attending clinician.
- ‘TELP with antimicrobial ceiling’ describes a TELP that included an instruction not to prescribe antimicrobial treatment in the event of patient deterioration. (Importantly, the TELP does not mandate treatment withdrawal.)
Statistical analysis
Chi-squared and Fisher’s Exact tests were used to determine the significance of differences in proportions. One-way analysis of variance was used to explore relationships between the number of antimicrobials prescribed, admission length (days) and the frequency of adverse events. Possible correlations were determined by linear regression analyses.
Ethics
This audit comprised only a retrospective case-note review. Based on Health Research Authority criteria, Ethics Committee approval was not required. All data were anonymised.
Results
Hospital records for 94 deceased patients were obtained (47 males). The mean age at time of death was 73.7 years (range: 53–96 years). The median length of stay was 14 days (range: 1–67 days). Eighty one of the 94 patients (86.2%) had a TELP including do not attempt cardiopulmonary resuscitation (DNACPR), nine (9.6%) had a DNACPR only and four (4.3%) had neither. Using GSF PIG criteria, 82 out of 94 patients (87.2%) were identified as having an ‘expected’ death.
Use of antimicrobials
Seventy two of 94 patients (76.6%) received antimicrobials during their final admission and 23 (24.5%) were receiving antimicrobials at time of death. The median number of antimicrobials per patient was two. There was a highly significant correlation between length of stay and the number of antimicrobials prescribed (r = 0.30; p = 0.004). Among the 72 patients, 44 (61.1%) had either a positive culture result (n = 15) or indirect evidence of infection (n = 29).
‘Inappropriate’ use of antimicrobials in relation to clinical decision-making
Criterion 1: Among the 81 out of 94 patients with a TELP (86.1%), antimicrobials were prescribed more frequently where the TELP did not include an antimicrobial ‘ceiling’ (43 out of 53; 81.1%) than for eight out of 28 (28.6%) among those with an antimicrobial ‘ceiling’ (p < 0.0005). Similarly, among those with a ‘ceiling’, two out of 28 (7.1%) received antimicrobials on the day of death compared to 18 out of 53 (34.0%) among those without a ‘ceiling’ (p = 0.01; Figure 1).
Figure 1 A cascade diagram depicting the numbers of patients who had a TELP with or without an antimicrobial ‘ceiling’, and in whom death was subsequently assessed to be imminent. The series of figures at the far right is the number of patients who were/were not receiving antimicrobials on the last day of life. N/A: not applicable; TELP: treatment escalation/limitation plan
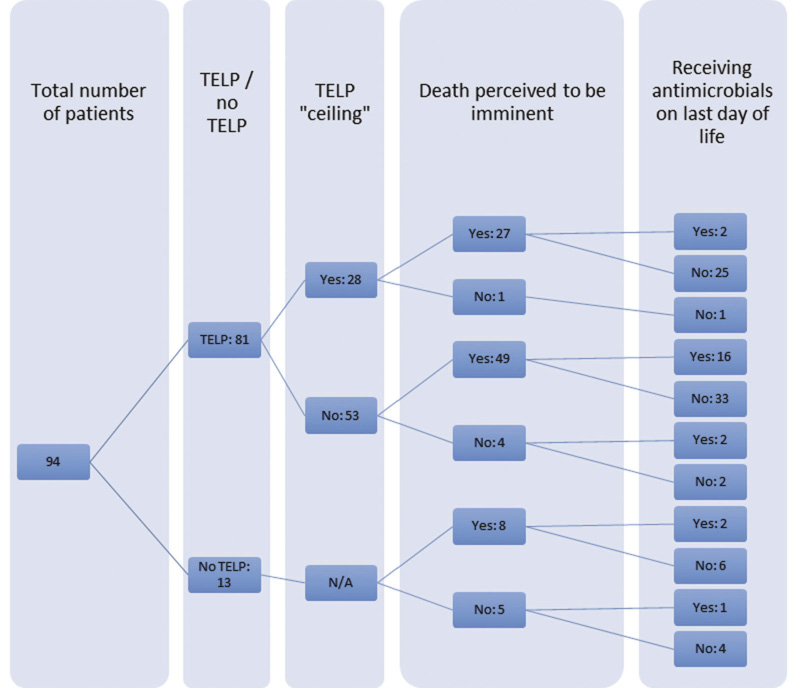
Criterion 2: In 84 out of 94 cases (89.4%) death was recognised as imminent by clinicians. Twenty of these 84 (23.8%) were receiving antimicrobials on the day of death. This was not significantly differently from the three out of 10 (30%) whose death was not recognised as imminent.
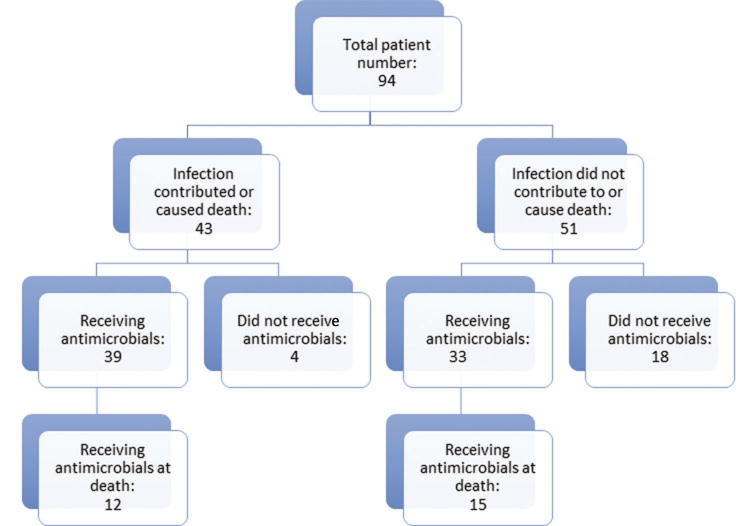
Criterion 3: Among the 43 out of 94 (45.7%) patients in whom infection was considered to be a contributor to or a cause of death, there was direct evidence of infection in 11 and indirect evidence in 22 [total = 33 (76.7%)]. Thirty nine out of 43 (90.7%) received antimicrobials during their admission. Among the 51 out of 94 patients (54.3%) in whom infection was considered noncontributory, 33 out of 51 (64.7%) received antimicrobials, including 15 out of 51 (29.4%) at the time of death (Figure 2). Conversely, among the 72 patients who received antimicrobials at any time, infection was not considered to be a contributor to or a cause of death in 33 out of 72 (45.8%).
Figure 2 A cascade diagram depicting the numbers of patients in whom infection did or did not contribute to death, and whether or not they received antimicrobials at any point during their final hospital admission and on the day of death
Adverse effects, including antimicrobial resistance
The percentage of patients who received none, one, two, three, or four or more antimicrobials were 23.4%, 18.1%, 27.7%, 17.0% and 13.8%, respectively. Adverse events related to antimicrobials occurred in 10 out of 72 (13.9%) and were mild in six and moderate to severe in four. There was a highly significant correlation between the number of antimicrobials prescribed and the frequency of adverse events (r = 0.50; p < 0.001). Similarly, antimicrobial resistance (AMR) was significantly associated with the number of antimicrobials (p < 0.01).
Among 40 patients in whom positive cultures were obtained, a resistant species was identified in eight out of 40 (20%). The median number of antimicrobials prescribed in this group was three, whereas among those with antimicrobial-susceptible cultures (n = 32) the median was two. Among the eight patients with AMR, antimicrobial prescribing was deemed inappropriate in two using one or more of the a priori criteria.
Discussion
The principal findings in our audit were that antimicrobial prescribing was common and often inappropriate in patients who were at the end of life. Among our population of 94 patients, 72 (76.6%) received antimicrobials during their final admission, 23 (24.5%) on the day of death. This is higher than the previously reported point prevalence for antimicrobial prescribing in acute hospitals in Scotland (35.3%) and UHW (40.1%).15 Of the 72 patients, 33 (45.8%) did not have infection as a recognised cause of or contributor to death. In addition, 28 out of 72 (38.9%) received antimicrobials even although no evidence of infection was obtained. These data reflect the findings in other studies.16–18 Prescribing was largely unaffected by awareness that a patient was terminally ill. All of these results indicate the need for a more systematic and rational approach to antimicrobial prescribing in dying patients.
Prescribing was modified when a TELP13 was in place. As far as we are aware, this is the first report showing that a TELP has benefits in reducing inappropriate antimicrobial prescribing. The use of the TELP was common among our patients (86.2%). The NHS Lanarkshire TELP (Appendix 1) is designed to avoid treatment that is ‘futile, burdensome and/or contrary to the patient’s wishes’ in critically ill patients. Options in the TELP include: ‘Antibiotics – Yes/No’. Where a TELP specifically included an antimicrobial ‘ceiling’, this resulted in a significant reduction in inappropriate antimicrobial use. This was striking on the day of death when only two out of 28 patients (7.1%) with a TELP that included a ‘ceiling’ were receiving antimicrobial treatment. This compared with 18 out of 53 (34.0%) among those who did not have a ‘ceiling’. This highlights how initiating a TELP prompts a review of all treatments that may or may not be appropriate.
Eight patients received antimicrobials at the time of death even though the TELP had specified that they should not be prescribed. This may have been because in response to a changing situation, the TELP had been redrafted (this is recommended practice) but antimicrobials already being given were not discontinued. Alternatively, there was possible nonadherence with the TELP and this raises concerns.
Antimicrobial therapy was continued in 22 out of 84 patients (26.2%) whose death was identified as imminent/inevitable (‘diagnosing dying’). This outcome may reflect clinicians’ reluctance to withdraw antimicrobial treatment in terminally ill patients. Even when there was no laboratory evidence of infection or when infection was not considered to be a contributor to a patient’s death, 23.8% of these patients were receiving antimicrobials on the day of death. These observations suggest that it is better not to start antimicrobials unless there is a specific indication. A TELP facilitates this outcome.
All of our findings raise broader questions as to why clinicians prescribe antimicrobials, often inappropriately, to dying patients. One of the relevant issues is the ‘goal of treatment’. Helde-Frankling et al.10 have claimed that terminally ill patients benefit from antimicrobial treatment even if no infection is identified. If the goal is to palliate symptoms that may or may not be due to infection-related cytokine release, then alternatives to antimicrobials are more appropriate.7,19 Another study concluded that antimicrobial treatment prolonged life but did not improve comfort, but this begs the question ‘What is the goal of treatment?’ in the context of caring for a dying patient.20
Whether or not there is curative or palliative intent or because withdrawing treatment is difficult, it is vital that appropriate reasons for antimicrobial prescribing should be applied. Futile antimicrobial treatment is potentially harmful as well as costly.21 Apart from adverse effects in individual patients, there is also the problem of AMR.18 Reducing antimicrobial use reduces AMR.22–25 Our results provide support that diagnosing dying, shared decision-making and the use of a TELP that limits unwarranted antimicrobial use, may serve as an adjunct to reducing the risk of AMR.26,27
In conclusion, antimicrobial treatment at the end of life is common. Neither the absence of evidence of infection nor recognising that a patient’s death is imminent appear to reduce antimicrobial use. However, using a TELP that includes explicit provision for limiting antimicrobial therapy reduces inappropriate prescribing and has the potential to reduce the risk of AMR. Implementing a TELP is best facilitated by discussing and agreeing patient-centred goals of care in critical illness.
Acknowledgements
The authors wish to thank Dr Margaret MacDougall for statistical advice and the secretarial staff of Ward 7, UHW, for facilitating the availability of hospital notes. The audit was conducted as part of a Student Selected Component Project in the University of Edinburgh Medical School (AW-S).
Online Supplementary Material
Appendix 1 is available with the online version of this paper, which can be accessed at https://www.rcpe.ac.uk/journal.
References
1 Clark D, Armstrong M, Allan A et al. Imminence of death among hospital inpatients: prevalence cohort study. Palliat Med 2014; 28: 474–9.
2 Cardona-Morrell M, Kim J, Turner RM et al. Non-beneficial treatments in hospital at the end of life: a systematic review on extent of the problem. Int J Qual Health Care 2016; 28: 456–69.
3 Carter HE, Winch S, Barnett AG et al. Incidence, duration and cost of futile treatment in end-of-life hospital admissions. BMJ Open 2017; 27: 261–5.
4 Guardian Newspaper. 13 October 2017.
5 Davies SC. Annual Report of the Chief Medical Officer. Volume 2. London: Department of Health London; 2011.
6 Furuno JP, Noble BN, Fromme EK. Should we refrain from antibiotic use in hospice patients? Expert Rev Anti Infect Ther 2016; 14: 277–80.
7 Rosenberg JH, Albrecht JS, Fromme EK. Antimicrobial use for symptom management in patients receiving hospice and palliative care: a systematic review. J Pall Med 2013; 16: 1568–74.
8 Chun ED, Rodgers PE, Vitale CA et al. Antimicrobial use in patients receiving palliative care. Am J Hospice Pall Med 2010; 27: 261–5.
9 Levin PD, Simor AE, Moses AE et al. End-of-life treatment and bacterial antibiotic resistance. Chest 2010; 138: 588–94.
10 Helde-Frankling M, Berqvist J, Bergman P et al. Antibiotic treatment in end-of-life cancer patients. Cancer 2016; 8: 84–94.
11 White PH, Kuhlenschmidt HL, Vancura BG et al. Antimicrobial use in patients with advanced cancer receiving hospice care. J Pain Symptom Manage 2003; 25: 438–43.
12 Stiel S, Krumm N, Pestinger M et al. Antibiotics in palliative medicine – results from a prospective epidemiological investigation from the HOPE survey. Support Care Cancer 2012; 20: 325–33.
13 Lightbody CJ, Campbell JN, Herbison GP et al. The impact of a treatment escalation/limitation plan on non-beneficial interventions and harms in patients during their last admission before in-hospital death, using the Structured Judgment Review Method. BMJ Open 2018; 8: e024264.
14 Thomas K, Wilson JA. Gold Standards Framework: Prognostic Indicator Guidance for clinicians to support earlier recognition of patients nearing the end of life. 2016. https://www.goldstandardsframework.org.uk/cd-content/uploads/files/Gener... (accessed 02/08/19).
15 Health Protection Scotland. National Point Prevalence Survey of Healthcare Associated Infection and Antimicrobial Prescribing. 2016. https://www.hps.scot.nhs.uk/resourcedocument.aspx?id=5964 (accessed 25/01/19).
16 Al-Shaqi MA, Alami AH, Al-Zahrani AS et al. The pattern of anti-microbial use for palliative care in-patients during the last week of life. Am J Hospice Pall Med 2012; 29: 60–3.
17 Macedo F, Nunes C, Ladeira K et al. Antimicrobial therapy in palliative care: an overview. Support Care Cancer 2018; 26: 1361–7.
18 Mitchell SL, Shaffer ML, Loeb MR et al. Infection management and multi-drug resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med 2014; 174: 1660–7.
19 Delia RV, Harrison K, Oyston PC et al. Targeting the cytokine storm for therapeutic benefit. Clin Vaccine Immunol 2013; 20: 319–27.
20 Servid SA, Noble BN, Fromme EK et al. Clinical intentions of antibiotics prescribed upon discharge to hospice care. J Am Geriatr Soc 2018; 66: 565–9.
21 Taylor DR, Lightbody CJ. Futility and appropriateness: challenging words, important concepts. Postgrad Med J 2018; 94: 238–43.
22 O’Neill J. Tackling drug resistant infections globally: final report and recommendations. The Review on Antimicrobial Resistance. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20c... (accessed 02/08/19).
23 Datta R, Juthani-Mehta M. Burden and management of multi-drug resistant organisms in palliative care. Pall Care 2017; 10: 1178224217749233.
24 Dancer SJ, Kirkatrick P, Corcoran DS et al. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital acquired C. difficile. Int J Antimicrob Agents 2013; 41: 137–42.
25 Seppala H. Klaukka T, Vuopio-Varkila J et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med 1997; 337: 441–6.
26 Global Action on antimicrobial resistance. World Health Organisation. 2015. http://www.who.int/drugresistance/global_action_plan/en (accessed 19/11/18).
27 Davey P, Marwick CA, Scott CL et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; CD003543.